Fairer AI in ophthalmology via implicit fairness learning for mitigating sexism and ageism
- PMID: 38834557
- PMCID: PMC11150422
- DOI: 10.1038/s41467-024-48972-0
Fairer AI in ophthalmology via implicit fairness learning for mitigating sexism and ageism
Abstract
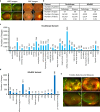
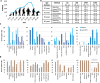
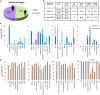
The transformative role of artificial intelligence (AI) in various fields highlights the need for it to be both accurate and fair. Biased medical AI systems pose significant potential risks to achieving fair and equitable healthcare. Here, we show an implicit fairness learning approach to build a fairer ophthalmology AI (called FairerOPTH) that mitigates sex (biological attribute) and age biases in AI diagnosis of eye diseases. Specifically, FairerOPTH incorporates the causal relationship between fundus features and eye diseases, which is relatively independent of sensitive attributes such as race, sex, and age. We demonstrate on a large and diverse collected dataset that FairerOPTH significantly outperforms several state-of-the-art approaches in terms of diagnostic accuracy and fairness for 38 eye diseases in ultra-widefield imaging and 16 eye diseases in narrow-angle imaging. This work demonstrates the significant potential of implicit fairness learning in promoting equitable treatment for patients regardless of their sex or age.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Digital Ageism: Challenges and Opportunities in Artificial Intelligence for Older Adults.Gerontologist. 2022 Aug 12;62(7):947-955. doi: 10.1093/geront/gnab167. Gerontologist. 2022. PMID: 35048111 Free PMC article.
-
[Challenges and prospects in the application of artificial intelligence for ocular disease screening and diagnosis].Zhonghua Yan Ke Za Zhi. 2024 Jun 11;60(6):484-489. doi: 10.3760/cma.j.cn112142-20231111-00226. Zhonghua Yan Ke Za Zhi. 2024. PMID: 38825947 Chinese.
-
Fairness of artificial intelligence in healthcare: review and recommendations.Jpn J Radiol. 2024 Jan;42(1):3-15. doi: 10.1007/s11604-023-01474-3. Epub 2023 Aug 4. Jpn J Radiol. 2024. PMID: 37540463 Free PMC article. Review.
-
AI in Neuro-Ophthalmology: Current Practice and Future Opportunities.J Neuroophthalmol. 2024 Sep 1;44(3):308-318. doi: 10.1097/WNO.0000000000002205. Epub 2024 Jul 5. J Neuroophthalmol. 2024. PMID: 38965655 Review.
-
Advancements and turning point of artificial intelligence in ophthalmology: A comprehensive analysis of research trends and collaborative networks.Ophthalmic Physiol Opt. 2024 Jul;44(5):1031-1040. doi: 10.1111/opo.13315. Epub 2024 Apr 6. Ophthalmic Physiol Opt. 2024. PMID: 38581209 Review.
Cited by
-
Towards machine learning fairness in classifying multicategory causes of deaths in colorectal or lung cancer patients.bioRxiv [Preprint]. 2025 Feb 19:2025.02.14.638368. doi: 10.1101/2025.02.14.638368. bioRxiv. 2025. Update in: Brief Bioinform. 2025 Jul 2;26(4):bbaf398. doi: 10.1093/bib/bbaf398. PMID: 40027644 Free PMC article. Updated. Preprint.
-
Ves-GAN: Unsupervised Vessel-Targeted Low-Dose Coronary Computed Tomography Angiography Denoising Framework.BME Front. 2025 Jul 4;6:0149. doi: 10.34133/bmef.0149. eCollection 2025. BME Front. 2025. PMID: 40625644 Free PMC article.
-
Towards machine learning fairness in classifying multicategory causes of deaths in colorectal or lung cancer patients.Brief Bioinform. 2025 Jul 2;26(4):bbaf398. doi: 10.1093/bib/bbaf398. Brief Bioinform. 2025. PMID: 40794953 Free PMC article.
References
-
- Du, S. et al. FairDisCo: Fairer AI in Dermatology via Disentanglement Contrastive Learning. In ECCV Workshops (Springer, Cham, 2022).
-
- Du M, et al. Fairness in deep learning: a computational perspective. IEEE Intell. Syst. 2020;36:25–34. doi: 10.1109/MIS.2020.3000681. - DOI
MeSH terms
Grants and funding
- U2001209/National Natural Science Foundation of China (National Science Foundation of China)
- 21ZR1406600/Natural Science Foundation of Shanghai (Natural Science Foundation of Shanghai Municipality)
- 62372117/National Natural Science Foundation of China (National Science Foundation of China)
- 82020108006/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

