Bacteriophage defends murine gut from Escherichia coli invasion via mucosal adherence
- PMID: 38834561
- PMCID: PMC11150434
- DOI: 10.1038/s41467-024-48560-2
Bacteriophage defends murine gut from Escherichia coli invasion via mucosal adherence
Abstract
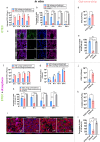
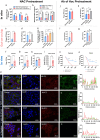
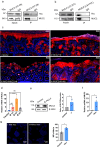
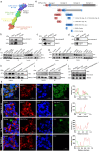
Bacteriophage are sophisticated cellular parasites that can not only parasitize bacteria but are increasingly recognized for their direct interactions with mammalian hosts. Phage adherence to mucus is known to mediate enhanced antimicrobial effects in vitro. However, little is known about the therapeutic efficacy of mucus-adherent phages in vivo. Here, using a combination of in vitro gastrointestinal cell lines, a gut-on-a-chip microfluidic model, and an in vivo murine gut model, we demonstrated that a E. coli phage, øPNJ-6, provided enhanced gastrointestinal persistence and antimicrobial effects. øPNJ-6 bound fucose residues, of the gut secreted glycoprotein MUC2, through domain 1 of its Hoc protein, which led to increased intestinal mucus production that was suggestive of a positive feedback loop mediated by the mucus-adherent phage. These findings extend the Bacteriophage Adherence to Mucus model into phage therapy, demonstrating that øPNJ-6 displays enhanced persistence within the murine gut, leading to targeted depletion of intestinal pathogenic bacteria.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

