Influence of heterochirality on the structure, dynamics, biological properties of cyclic(PFPF) tetrapeptides obtained by solvent-free ball mill mechanosynthesis
- PMID: 38834643
- PMCID: PMC11150477
- DOI: 10.1038/s41598-024-63552-4
Influence of heterochirality on the structure, dynamics, biological properties of cyclic(PFPF) tetrapeptides obtained by solvent-free ball mill mechanosynthesis
Abstract
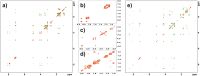
Cyclic tetrapeptides c(Pro-Phe-Pro-Phe) obtained by the mechanosynthetic method using a ball mill were isolated in a pure stereochemical form as a homochiral system (all L-amino acids, sample A) and as a heterochiral system with D configuration at one of the stereogenic centers of Phe (sample B). The structure and stereochemistry of both samples were determined by X-ray diffraction studies of single crystals. In DMSO and acetonitrile, sample A exists as an equimolar mixture of two conformers, while only one is monitored for sample B. The conformational space and energetic preferences for possible conformers were calculated using DFT methods. The distinctly different conformational flexibility of the two samples was experimentally proven by Variable Temperature (VT) and 2D EXSY NMR measurements. Both samples were docked to histone deacetylase HDAC8. Cytotoxic studies proved that none of the tested cyclic peptide is toxic.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









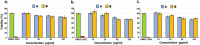
References
-
- Empting M. CHAPTER 1. An introduction to cyclic peptides. In: Koehnke J, Naismith J, Van Der Donk WA, editors. Chemical Biology. Cambridge: Royal Society of Chemistry; 2017. pp. 1–14.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

