A culture method with berbamine, a plant alkaloid, enhances CAR-T cell efficacy through modulating cellular metabolism
- PMID: 38834758
- PMCID: PMC11150386
- DOI: 10.1038/s42003-024-06297-0
A culture method with berbamine, a plant alkaloid, enhances CAR-T cell efficacy through modulating cellular metabolism
Abstract
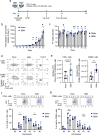
Memory T cells demonstrate superior in vivo persistence and antitumor efficacy. However, methods for manufacturing less differentiated T cells are not yet well-established. Here, we show that producing chimeric antigen receptor (CAR)-T cells using berbamine (BBM), a natural compound found in the Chinese herbal medicine Berberis amurensis, enhances the antitumor efficacy of CAR-T cells. BBM is identified through cell-based screening of chemical compounds using induced pluripotent stem cell-derived T cells, leading to improved viability with a memory T cell phenotype. Transcriptomics and metabolomics using stem cell memory T cells reveal that BBM broadly enhances lipid metabolism. Furthermore, the addition of BBM downregulates the phosphorylation of p38 mitogen-activated protein kinase and enhanced mitochondrial respiration. CD19-CAR-T cells cultured with BBM also extend the survival of leukaemia mouse models due to their superior in vivo persistence. This technology offers a straightforward approach to enhancing the antitumor efficacy of CAR-T cells.
© 2024. The Author(s).
Conflict of interest statement
S-i.T., S.C., K.F., A.K., Y.Kawai, and Sh.K. declare a patent titled Production method for T cells or NK cells, medium for culturing T cells or NK cells, method for culturing T cells or NK cells, method for maintaining undifferentiated state of undifferentiated T cells, and growth-accelerating agent for T cells or NK cells’ (WO2020116606) related to this work. S-i.T., S.C., S.H., K.F., N.W., N.O., Y.Kurimoto., A.F., A.K., M.K., and H.N. are employed by Kirin Holdings, Co., Ltd. Sh.K. is a founder, shareholder, and board member at Shinobi Therapeutics, Inc., and received research fundings from Kirin Holdings, Co., Ltd., Takeda Pharmaceutical Co., Ltd., Shinobi Therapeutics, Inc., Astellas Co., Ltd., Terumo Co., Ltd., and Tosoh Co., Ltd. The remaining authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

