PARP14 is regulated by the PARP9/DTX3L complex and promotes interferon γ-induced ADP-ribosylation
- PMID: 38834852
- PMCID: PMC11251048
- DOI: 10.1038/s44318-024-00125-1
PARP14 is regulated by the PARP9/DTX3L complex and promotes interferon γ-induced ADP-ribosylation
Abstract
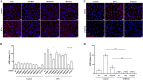
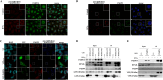
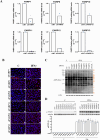
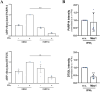
Protein ADP-ribosylation plays important but ill-defined roles in antiviral signalling cascades such as the interferon response. Several viruses of clinical interest, including coronaviruses, express hydrolases that reverse ADP-ribosylation catalysed by host enzymes, suggesting an important role for this modification in host-pathogen interactions. However, which ADP-ribosyltransferases mediate host ADP-ribosylation, what proteins and pathways they target and how these modifications affect viral infection and pathogenesis is currently unclear. Here we show that host ADP-ribosyltransferase activity induced by IFNγ signalling depends on PARP14 catalytic activity and that the PARP9/DTX3L complex is required to uphold PARP14 protein levels via post-translational mechanisms. Both the PARP9/DTX3L complex and PARP14 localise to IFNγ-induced cytoplasmic inclusions containing ADP-ribosylated proteins, and both PARP14 itself and DTX3L are likely targets of PARP14 ADP-ribosylation. We provide evidence that these modifications are hydrolysed by the SARS-CoV-2 Nsp3 macrodomain, shedding light on the intricate cross-regulation between IFN-induced ADP-ribosyltransferases and the potential roles of the coronavirus macrodomain in counteracting their activity.
Keywords: ADP-Ribosylation; Coronavirus; Innate Immunity; Interferon; PARP.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Abraham R, Hauer D, McPherson RL, Utt A, Kirby IT, Cohen MS, Merits A, Leung AKL, Griffin DE. ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc Natl Acad Sci USA. 2018;115:E10457–E10466. doi: 10.1073/pnas.1812130115. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2018/18007-5/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2020/05317-6/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2019/25914-1/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2020/11162-5 and 2022/10947-4/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2019/06039-2/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
LinkOut - more resources
Full Text Sources
Miscellaneous

