Cost-Effectiveness Analysis of Maternal Respiratory Syncytial Virus Vaccine in Protecting Infants from RSV Infection in Japan
- PMID: 38834858
- PMCID: PMC11219667
- DOI: 10.1007/s40121-024-01000-6
Cost-Effectiveness Analysis of Maternal Respiratory Syncytial Virus Vaccine in Protecting Infants from RSV Infection in Japan
Abstract
Introduction: Respiratory syncytial virus (RSV) is one of the major causes of respiratory tract infections among children. Until recently, the monoclonal antibody palivizumab was the only RSV prophylaxis available in Japan. In 2024, the bivalent RSV prefusion F protein-based (RSVpreF) vaccine was approved for the prevention of RSV infection in infants by active immunization of pregnant women. In this study, we assessed the cost-effectiveness of a combined strategy of RSVpreF vaccine and palivizumab in Japanese setting.
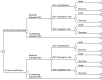
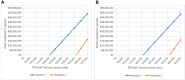
Methods: Using a Markov model, we evaluated prevented cases and deaths of medically attended RSV infections from birth to age 11 months for each of the three healthcare settings: inpatient (hospitalization), emergency department visits, and outpatient visits. Incremental cost-effectiveness ratios (ICERs) were calculated from economic outcomes (intervention costs, medication costs, and productivity losses) and quality-adjusted life years (QALYs). Further, we calculated the maximum price of RSVpreF vaccine within which the program would be cost-effective.
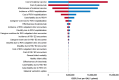
Results: In comparison with the current prophylaxis (palivizumab alone), a combined prophylaxis of year-round RSVpreF vaccination of pregnant women and palivizumab prescription for premature infants born in < 32 weeks gestational age (wGA) and all infants with high risk prevented 14,382 medically attended cases of RSV (hospitalization, 7490 cases; emergency department, 2239 cases; outpatient, 4653 cases) and 7 deaths, respectively. From a healthcare payer perspective, when the price of RSVpreF vaccine was equal to or less than ¥23,948 (US $182), a combination prophylaxis was cost-effective under the ICER threshold of ¥5 million per QALY. The other combination prophylaxis of year-round RSVpreF vaccination and palivizumab prescription of premature born in < 32 wGA regardless of risk in infants was a dominant strategy (more effective and less costly).
Conclusion: A combined prophylaxis of year-round RSVpreF vaccine and palivizumab could be a cost-effective strategy to protect neonates throughout the infant stage (< 1 years old) in Japan.
Keywords: Cost-effectiveness analysis; Japan; Palivizumab; RSVpreF; Respiratory syncytial virus; Vaccination.
© 2024. The Author(s).
Conflict of interest statement
Naruhiko Ishiwada has received funding for research and honoraria for lectures and consultancy from Pfizer Japan Inc. and funding for research from MSD K.K., Sanofi K.K. and Shionogi & Co., Ltd. Rina Akaishi has received honoraria for consultancy from Pfizer Japan Inc. Kanae Togo, Yasuhiro Kobayashi, Naohiro Yonemoto and Kazumasa Kamei are employees of Pfizer Japan Inc. Moe Matsuo and Shinnosuke Kaneko are an employee of Syneos Health Clinical K.K. Amy W. Law is an employee of Pfizer Inc.
Figures
Similar articles
-
Cost-effectiveness of bivalent respiratory syncytial virus Prefusion F (RSVpreF) maternal vaccine among infants in the United States.Vaccine. 2025 Jun 11;58:127191. doi: 10.1016/j.vaccine.2025.127191. Epub 2025 May 17. Vaccine. 2025. PMID: 40383082
-
Cost-effectiveness of RSVpreF vaccine and nirsevimab for the prevention of respiratory syncytial virus disease in Canadian infants.Vaccine. 2024 Aug 30;42(21):126164. doi: 10.1016/j.vaccine.2024.126164. Epub 2024 Jul 30. Vaccine. 2024. PMID: 39079810
-
Burden of respiratory syncytial virus disease in infants and the potential value of maternal immunization in Greece.Front Public Health. 2025 Jul 16;13:1611483. doi: 10.3389/fpubh.2025.1611483. eCollection 2025. Front Public Health. 2025. PMID: 40740353 Free PMC article.
-
Updated cost-effectiveness analysis of palivizumab (Synagis) for the prophylaxis of respiratory syncytial virus in infant populations in the UK.J Med Econ. 2020 Dec;23(12):1640-1652. doi: 10.1080/13696998.2020.1836923. Epub 2020 Oct 27. J Med Econ. 2020. PMID: 33107769 Review.
-
Respiratory Syncytial Virus Prefusion F Subunit Vaccine: First Approval of a Maternal Vaccine to Protect Infants.Paediatr Drugs. 2023 Nov;25(6):729-734. doi: 10.1007/s40272-023-00598-3. Paediatr Drugs. 2023. PMID: 37831328 Review.
Cited by
-
Complementary Strategy of Maternal Immunization with RSVpreF Vaccine and Monoclonal Antibodies for the Prevention of Respiratory Syncytial Virus Among Italian Infants: A Cost-Effectiveness Assessment.Infect Dis Ther. 2025 Aug;14(8):1883-1897. doi: 10.1007/s40121-025-01193-4. Epub 2025 Jul 18. Infect Dis Ther. 2025. PMID: 40679786 Free PMC article.
-
Cost-Effectiveness Analysis of a Bivalent RSVPreF Vaccine in Japanese Adults Aged 60 Years and Older.Infect Dis Ther. 2025 Aug;14(8):1755-1773. doi: 10.1007/s40121-025-01177-4. Epub 2025 Jul 6. Infect Dis Ther. 2025. PMID: 40618285 Free PMC article.
-
Respiratory Syncytial Virus: A Narrative Review of Updates and Recent Advances in Epidemiology, Pathogenesis, Diagnosis, Management and Prevention.J Clin Med. 2025 May 30;14(11):3880. doi: 10.3390/jcm14113880. J Clin Med. 2025. PMID: 40507642 Free PMC article. Review.
References
-
- Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. - PubMed
LinkOut - more resources
Full Text Sources

