Cost-Effectiveness Analysis of Maternal Respiratory Syncytial Virus Vaccine in Protecting Infants from RSV Infection in Japan
- PMID: 38834858
- PMCID: PMC11219667
- DOI: 10.1007/s40121-024-01000-6
Cost-Effectiveness Analysis of Maternal Respiratory Syncytial Virus Vaccine in Protecting Infants from RSV Infection in Japan
Abstract
Introduction: Respiratory syncytial virus (RSV) is one of the major causes of respiratory tract infections among children. Until recently, the monoclonal antibody palivizumab was the only RSV prophylaxis available in Japan. In 2024, the bivalent RSV prefusion F protein-based (RSVpreF) vaccine was approved for the prevention of RSV infection in infants by active immunization of pregnant women. In this study, we assessed the cost-effectiveness of a combined strategy of RSVpreF vaccine and palivizumab in Japanese setting.
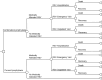
Methods: Using a Markov model, we evaluated prevented cases and deaths of medically attended RSV infections from birth to age 11 months for each of the three healthcare settings: inpatient (hospitalization), emergency department visits, and outpatient visits. Incremental cost-effectiveness ratios (ICERs) were calculated from economic outcomes (intervention costs, medication costs, and productivity losses) and quality-adjusted life years (QALYs). Further, we calculated the maximum price of RSVpreF vaccine within which the program would be cost-effective.
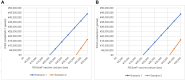
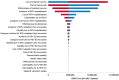
Results: In comparison with the current prophylaxis (palivizumab alone), a combined prophylaxis of year-round RSVpreF vaccination of pregnant women and palivizumab prescription for premature infants born in < 32 weeks gestational age (wGA) and all infants with high risk prevented 14,382 medically attended cases of RSV (hospitalization, 7490 cases; emergency department, 2239 cases; outpatient, 4653 cases) and 7 deaths, respectively. From a healthcare payer perspective, when the price of RSVpreF vaccine was equal to or less than ¥23,948 (US $182), a combination prophylaxis was cost-effective under the ICER threshold of ¥5 million per QALY. The other combination prophylaxis of year-round RSVpreF vaccination and palivizumab prescription of premature born in < 32 wGA regardless of risk in infants was a dominant strategy (more effective and less costly).
Conclusion: A combined prophylaxis of year-round RSVpreF vaccine and palivizumab could be a cost-effective strategy to protect neonates throughout the infant stage (< 1 years old) in Japan.
Keywords: Cost-effectiveness analysis; Japan; Palivizumab; RSVpreF; Respiratory syncytial virus; Vaccination.
© 2024. The Author(s).
Conflict of interest statement
Naruhiko Ishiwada has received funding for research and honoraria for lectures and consultancy from Pfizer Japan Inc. and funding for research from MSD K.K., Sanofi K.K. and Shionogi & Co., Ltd. Rina Akaishi has received honoraria for consultancy from Pfizer Japan Inc. Kanae Togo, Yasuhiro Kobayashi, Naohiro Yonemoto and Kazumasa Kamei are employees of Pfizer Japan Inc. Moe Matsuo and Shinnosuke Kaneko are an employee of Syneos Health Clinical K.K. Amy W. Law is an employee of Pfizer Inc.
Figures
References
-
- Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. - PubMed
LinkOut - more resources
Full Text Sources

