Sperm epigenetics and male infertility: unraveling the molecular puzzle
- PMID: 38835100
- PMCID: PMC11149391
- DOI: 10.1186/s40246-024-00626-4
Sperm epigenetics and male infertility: unraveling the molecular puzzle
Abstract
Background: The prevalence of infertility among couples is estimated to range from 8 to 12%. A paradigm shift has occurred in understanding of infertility, challenging the notion that it predominantly affects women. It is now acknowledged that a significant proportion, if not the majority, of infertility cases can be attributed to male-related factors. Various elements contribute to male reproductive impairments, including aberrant sperm production caused by pituitary malfunction, testicular malignancies, aplastic germ cells, varicocele, and environmental factors.
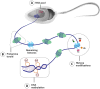
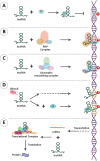
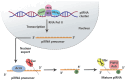
Main body: The epigenetic profile of mammalian sperm is distinctive and specialized. Various epigenetic factors regulate genes across different levels in sperm, thereby affecting its function. Changes in sperm epigenetics, potentially influenced by factors such as environmental exposures, could contribute to the development of male infertility.
Conclusion: In conclusion, this review investigates the latest studies pertaining to the mechanisms of epigenetic changes that occur in sperm cells and their association with male reproductive issues.
Keywords: DNA methylation; Epigenetics; Histone modifications; Infertility; Non-coding RNAs; Sperm.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Male infertility |. Nature Reviews Disease Primers [Internet]. [cited 2023 Oct 13]. https://www.nature.com/articles/s41572-023-00459-w.
-
- Leslie SW, Soon-Sutton TL, Khan MA. Male Infertility. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Oct 13]. http://www.ncbi.nlm.nih.gov/books/NBK562258/.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

