Distinctive contribution of two additional residues in protein aggregation of Aβ42 and Aβ40 isoforms
- PMID: 38835114
- PMCID: PMC11214890
- DOI: 10.5483/BMBRep.2024-0044
Distinctive contribution of two additional residues in protein aggregation of Aβ42 and Aβ40 isoforms
Abstract
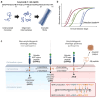
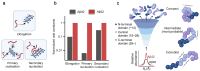
Amyloid-β (Aβ) is one of the amyloidogenic intrinsically disordered proteins (IDPs) that self-assemble to protein aggregates, incurring cell malfunction and cytotoxicity. While Aβ has been known to regulate multiple physiological functions, such as enhancing synaptic functions, aiding in the recovery of the blood-brain barrier/brain injury, and exhibiting tumor suppression/antimicrobial activities, the hydrophobicity of the primary structure promotes pathological aggregations that are closely associated with the onset of Alzheimer's disease (AD). Aβ proteins consist of multiple isoforms with 37-43 amino acid residues that are produced by the cleavage of amyloid-β precursor protein (APP). The hydrolytic products of APP are secreted to the extracellular regions of neuronal cells. Aβ 1-42 (Aβ42) and Aβ 1-40 (Aβ40) are dominant isoforms whose significance in AD pathogenesis has been highlighted in numerous studies to understand the molecular mechanism and develop AD diagnosis and therapeutic strategies. In this review, we focus on the differences between Aβ42 and Aβ40 in the molecular mechanism of amyloid aggregations mediated by the two additional residues (Ile41 and Ala42) of Aβ42. The current comprehension of Aβ42 and Aβ40 in AD progression is outlined, together with the structural features of Aβ42/Aβ40 amyloid fibrils, and the aggregation mechanisms of Aβ42/Aβ40. Furthermore, the impact of the heterogeneous distribution of Aβ isoforms during amyloid aggregations is discussed in the system mimicking the coexistence of Aβ42 and Aβ40 in human cerebrospinal fluid (CSF) and plasma. [BMB Reports 2024; 57(6): 263-272].
Conflict of interest statement
The authors have no conflicting interests.
Figures

Similar articles
-
N-Terminus Binding Preference for Either Tanshinone or Analogue in Both Inhibition of Amyloid Aggregation and Disaggregation of Preformed Amyloid Fibrils-Toward Introducing a Kind of Novel Anti-Alzheimer Compounds.ACS Chem Neurosci. 2017 Jul 19;8(7):1577-1588. doi: 10.1021/acschemneuro.7b00080. Epub 2017 Apr 28. ACS Chem Neurosci. 2017. PMID: 28406293
-
Aβ42 and Aβ40: similarities and differences.J Pept Sci. 2015 Jul;21(7):522-9. doi: 10.1002/psc.2789. Epub 2015 May 28. J Pept Sci. 2015. PMID: 26018760 Review.
-
Cross-Interactions of Aβ Peptides Implicated in Alzheimer's Disease Shape Amyloid Oligomer Structures and Aggregation.ACS Chem Neurosci. 2024 Dec 4;15(23):4295-4304. doi: 10.1021/acschemneuro.4c00492. Epub 2024 Nov 19. ACS Chem Neurosci. 2024. PMID: 39561091
-
Amyloid-Beta Peptides 40 and 42 Employ Distinct Molecular Pathways for Cell Entry and Intracellular Transit at the Blood-Brain Barrier Endothelium.Mol Pharmacol. 2023 Nov;104(5):203-213. doi: 10.1124/molpharm.123.000670. Epub 2023 Aug 4. Mol Pharmacol. 2023. PMID: 37541759 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Mirodenafil improves cognitive function by reducing microglial activation and blood-brain barrier permeability in ApoE4 KI mice.Front Aging Neurosci. 2025 May 15;17:1579411. doi: 10.3389/fnagi.2025.1579411. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40443793 Free PMC article.
-
EBP1 potentiates amyloid β pathology by regulating γ-secretase.Nat Aging. 2025 Mar;5(3):486-503. doi: 10.1038/s43587-024-00790-1. Epub 2025 Jan 8. Nat Aging. 2025. PMID: 39779912
-
Dual modulation of amyloid beta and tau aggregation and dissociation in Alzheimer's disease: a comprehensive review of the characteristics and therapeutic strategies.Transl Neurodegener. 2025 Mar 26;14(1):15. doi: 10.1186/s40035-025-00479-4. Transl Neurodegener. 2025. PMID: 40133924 Free PMC article. Review.
-
Antiparallel β-Sheet as a Key Motif of Amyloid-β Inhibitor Designed via Topological Peptide Reprogramming.Angew Chem Int Ed Engl. 2025 Jul 7;64(28):e202504640. doi: 10.1002/anie.202504640. Epub 2025 May 22. Angew Chem Int Ed Engl. 2025. PMID: 40345176 Free PMC article.

