The economic rationale for cell-based influenza vaccines in children and adults: A review of cost-effectiveness analyses
- PMID: 38835218
- PMCID: PMC11155702
- DOI: 10.1080/21645515.2024.2351675
The economic rationale for cell-based influenza vaccines in children and adults: A review of cost-effectiveness analyses
Abstract
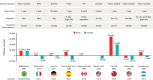
Seasonal influenza significantly affects both health and economic costs in children and adults. This narrative review summarizes published cost-effectiveness analyses (CEAs) of cell-based influenza vaccines in children and adults <65 years of age, critically assesses the assumptions and approaches used in these analyses, and considers the role of cell-based influenza vaccines for children and adults. CEAs from multiple countries demonstrated the cost-effectiveness of cell-based quadrivalent influenza vaccines (QIVc) compared with egg-based trivalent/quadrivalent influenza vaccines (TIVe/QIVe). CEA findings were consistent across models relying on different relative vaccine effectiveness (rVE) estimate inputs, with the rVE of QIVc versus QIVe ranging from 8.1% to 36.2% in favor of QIVc. Across multiple scenarios and types of analyses, QIVc was consistently cost-effective compared with QIVe, including in children and adults across different regions of the world.
Keywords: Influenza; cell-based influenza vaccine; cost-effectiveness analysis.
Conflict of interest statement
D.F. has received honoraria for participating in advisory boards with Merck, Sanofi-Pasteur, CSL Seqirus, Pfizer, and AstraZeneca related to influenza, SARS-CoV-2, and pneumococcal vaccines.
N. G. received funding for investigator-led studies from GSK, MSD, CSL Seqirus, Takeda, and Sanofi-Pasteur and Global Vaccine Data Network. N.G. has received honoraria from CSL Seqirus, Takeda, and Pfizer for acting as a speaker in congresses, and from GSK, Takeda, and CSL Seqirus for taking part in advisory boards.
M.J.L. received honoraria from CSL Seqirus, GSK, Pfizer, Moderna, Curevo, AstraZeneca, and Dynavax for taking part in advisory boards. M.J.L. has received institution funding for investigator-led studies from GSK.
V.H.N. received funding for conducting RWE and CEAs on vaccines from Takeda, CSL Seqirus, Pfizer, and Moderna. V.H.N. has also received honoraria from those companies for taking part in advisory boards.
S.I.P. has received honoraria from CSL Seqirus as a consultant on research design and analysis and participation in advisory boards on influenza vaccines.
M.P. has received honoraria from CSL Seqirus for taking part in advisory boards.
J.R.A. has received honoraria from CSL Seqirus, GSK, and MSD for taking part in advisory boards.
A.U. received institution funding for investigator-led studies from GSK, MSD, CSL Seqirus, Takeda, and Sanofi-Pasteur. A.U. has also received honoraria from CSL Seqirus and Takeda for acting as a speaker in congresses, and from GSK, Takeda, Sanofi, and CSL Seqirus for taking part in advisory boards. She is a member of the Directory board of Sociedad Argentina de Vacunología y Epidemiologia (SAVE).
J.M.Q. is an employee of CSL Seqirus.
Figures
Similar articles
-
A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017-18 influenza season.Vaccine. 2020 Sep 11;38(40):6334-6343. doi: 10.1016/j.vaccine.2020.07.023. Epub 2020 Jul 30. Vaccine. 2020. PMID: 32739119
-
Cost-effectiveness of cell-based influenza vaccine in France.Expert Rev Vaccines. 2024 Jan-Dec;23(1):1020-1028. doi: 10.1080/14760584.2024.2417854. Epub 2024 Oct 24. Expert Rev Vaccines. 2024. PMID: 39412212
-
Effectiveness of Cell-Based Quadrivalent Seasonal Influenza Vaccine: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2023 Oct 17;11(10):1607. doi: 10.3390/vaccines11101607. Vaccines (Basel). 2023. PMID: 37897009 Free PMC article. Review.
-
Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain.Hum Vaccin Immunother. 2020 Sep 1;16(9):2238-2244. doi: 10.1080/21645515.2020.1712935. Epub 2020 Feb 10. Hum Vaccin Immunother. 2020. PMID: 32040379 Free PMC article.
-
Cell-based influenza vaccines: An effective vaccine option for under 60-year-olds.GMS Hyg Infect Control. 2024 Apr 30;19:Doc21. doi: 10.3205/dgkh000476. eCollection 2024. GMS Hyg Infect Control. 2024. PMID: 38766639 Free PMC article. Review.
References
-
- World Health Organization . Influenza. https://www.who.int/teams/health-product-policy-and-standards/standards-....
-
- Paget WJ, Balderston C, Casas I, Donker G, Edelman L, Fleming D, Larrauri A, Meijer A, Puzelli S, Rizzo C, et al., EPIA collaborators . Assessing the burden of paediatric influenza in Europe: the European Paediatric Influenza Analysis (EPIA) project. Eur J Pediatr. 2010;169(8):997–1008. doi:10.1007/s00431-010-1164-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical