A circle of life: platelet and megakaryocyte cytoskeleton dynamics in health and disease
- PMID: 38835242
- PMCID: PMC11285727
- DOI: 10.1098/rsob.240041
A circle of life: platelet and megakaryocyte cytoskeleton dynamics in health and disease
Abstract
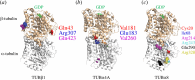
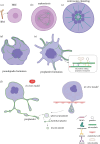
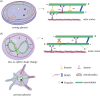
Platelets are blood cells derived from megakaryocytes that play a central role in regulating haemostasis and vascular integrity. The microtubule cytoskeleton of megakaryocytes undergoes a critical dynamic reorganization during cycles of endomitosis and platelet biogenesis. Quiescent platelets have a discoid shape maintained by a marginal band composed of microtubule bundles, which undergoes remarkable remodelling during platelet activation, driving shape change and platelet function. Disrupting or enhancing this process can cause platelet dysfunction such as bleeding disorders or thrombosis. However, little is known about the molecular mechanisms underlying the reorganization of the cytoskeleton in the platelet lineage. Recent studies indicate that the emergence of a unique platelet tubulin code and specific pathogenic tubulin mutations cause platelet defects and bleeding disorders. Frequently, these mutations exhibit dominant negative effects, offering valuable insights into both platelet disease mechanisms and the functioning of tubulins. This review will highlight our current understanding of the role of the microtubule cytoskeleton in the life and death of platelets, along with its relevance to platelet disorders.
Keywords: megakaryocyte; microtubule; motors; pathogenesis; platelet; tubulin.
Conflict of interest statement
We declare we have no competing interests.
Figures
Similar articles
-
Tubulin in Platelets: When the Shape Matters.Int J Mol Sci. 2019 Jul 16;20(14):3484. doi: 10.3390/ijms20143484. Int J Mol Sci. 2019. PMID: 31315202 Free PMC article. Review.
-
The tubulin code in platelet biogenesis.Semin Cell Dev Biol. 2023 Mar 15;137:63-73. doi: 10.1016/j.semcdb.2022.01.010. Epub 2022 Feb 9. Semin Cell Dev Biol. 2023. PMID: 35148939 Review.
-
Cytoskeletal regulation of platelet formation: Coordination of F-actin and microtubules.Int J Biochem Cell Biol. 2015 Sep;66:69-74. doi: 10.1016/j.biocel.2015.07.008. Epub 2015 Jul 23. Int J Biochem Cell Biol. 2015. PMID: 26210823 Review.
-
Formin proteins in megakaryocytes and platelets: regulation of actin and microtubule dynamics.Platelets. 2019;30(1):23-30. doi: 10.1080/09537104.2018.1481937. Epub 2018 Jun 18. Platelets. 2019. PMID: 29913076 Free PMC article. Review.
-
Visualization and manipulation of the platelet and megakaryocyte cytoskeleton.Methods Mol Biol. 2012;788:109-25. doi: 10.1007/978-1-61779-307-3_9. Methods Mol Biol. 2012. PMID: 22130704
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

