Virus-like particles expressing microneme-associated antigen of Plasmodium berghei confer better protection than those expressing apical membrane antigen 1
- PMID: 38835260
- PMCID: PMC11150920
- DOI: 10.3347/PHD.24017
Virus-like particles expressing microneme-associated antigen of Plasmodium berghei confer better protection than those expressing apical membrane antigen 1
Abstract
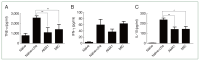
Malaria is a global disease affecting a large portion of the world's population. Although vaccines have recently become available, their efficacies are suboptimal. We generated virus-like particles (VLPs) that expressed either apical membrane antigen 1 (AMA1) or microneme-associated antigen (MIC) of Plasmodium berghei and compared their efficacy in BALB/c mice. We found that immune sera acquired from AMA1 VLP- or MIC VLP-immunized mice specifically interacted with the antigen of choice and the whole P. berghei lysate antigen, indicating that the antibodies were highly parasite-specific. Both VLP vaccines significantly enhanced germinal center B cell frequencies in the inguinal lymph nodes of mice compared with the control, but only the mice that received MIC VLPs showed significantly enhanced CD4+ T cell responses in the blood following P. berghei challenge infection. AMA1 and MIC VLPs significantly suppressed TNF-α and interleukin-10 production but had a negligible effect on interferon-γ. Both VLPs prevented excessive parasitemia buildup in immunized mice, although parasite burden reduction induced by MIC VLPs was slightly more effective than that induced by AMA1. Both VLPs were equally effective at preventing body weight loss. Our findings demonstrated that the MIC VLP was an effective inducer of protection against murine experimental malaria and should be the focus of further development.
Keywords: Plasmodium berghei; malaria; vaccine; virus-like particle.
Conflict of interest statement
Figures


Similar articles
-
Influenza virus-like particle vaccine containing both apical membrane antigen 1 and microneme-associated antigen proteins of Plasmodium berghei confers protection in mice.BMC Immunol. 2022 Apr 25;23(1):21. doi: 10.1186/s12865-022-00494-4. BMC Immunol. 2022. PMID: 35468726 Free PMC article.
-
CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization.J Immunol. 2000 Jul 1;165(1):389-96. doi: 10.4049/jimmunol.165.1.389. J Immunol. 2000. PMID: 10861076
-
Protection induced by malaria virus-like particles containing codon-optimized AMA-1 of Plasmodium berghei.Malar J. 2019 Dec 3;18(1):394. doi: 10.1186/s12936-019-3017-2. Malar J. 2019. PMID: 31796032 Free PMC article.
-
Apical membrane antigen 1: a malaria vaccine candidate in review.Trends Parasitol. 2008 Feb;24(2):74-84. doi: 10.1016/j.pt.2007.12.002. Epub 2008 Jan 15. Trends Parasitol. 2008. PMID: 18226584 Review.
-
Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria.Parasite Immunol. 2006 Jan-Feb;28(1-2):25-30. doi: 10.1111/j.1365-3024.2006.00767.x. Parasite Immunol. 2006. PMID: 16438673 Review.
Cited by
-
Ivermectin Identified Using a High-Throughput Screening System Exhibits Anti-Clonorchis sinensis Activity in Rats.Antibiotics (Basel). 2025 Aug 19;14(8):837. doi: 10.3390/antibiotics14080837. Antibiotics (Basel). 2025. PMID: 40868031 Free PMC article.
-
Protective Efficacy Induced by Virus-like Particles Expressing Dense Granule Protein 5 of Toxoplasma gondii.Vaccines (Basel). 2025 Jul 24;13(8):787. doi: 10.3390/vaccines13080787. Vaccines (Basel). 2025. PMID: 40872874 Free PMC article.
-
Orally Dissolving Film-Based Influenza Vaccines Confer Superior Protection Compared to the Oral Administration of Inactivated Influenza Virus.Vaccines (Basel). 2025 May 31;13(6):600. doi: 10.3390/vaccines13060600. Vaccines (Basel). 2025. PMID: 40573931 Free PMC article.
-
Efficacy of Heterologous Vaccination Using Virus-Like Particles and Vaccinia Virus Containing MIC8 and AMA1 Proteins of Toxoplasma gondii.Vaccines (Basel). 2025 Aug 15;13(8):862. doi: 10.3390/vaccines13080862. Vaccines (Basel). 2025. PMID: 40872947 Free PMC article.
References
-
- World Health Organization . World Malaria Report 2021. Geneva, Switzerland: World Health Organization. Geneva, Switzerland; 2021.
-
- Marwa K, Kapesa A, Baraka V, Konje E, Kidenya B, et al. Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2022;17(3):e0264339. doi: 10.1371/journal.pone.0264339. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

