Molecular cloning, identification, transcriptional analysis, and silencing of enolase on the life cycle of Haemaphysalis longicornis (Acari, Ixodidae) tick
- PMID: 38835263
- PMCID: PMC11150923
- DOI: 10.3347/PHD.24015
Molecular cloning, identification, transcriptional analysis, and silencing of enolase on the life cycle of Haemaphysalis longicornis (Acari, Ixodidae) tick
Abstract
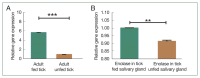
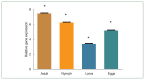
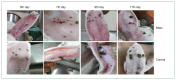
Ticks, blood-sucking ectoparasites, spread diseases to humans and animals. Haemaphysalis longicornis is a significant vector for tick-borne diseases in medical and veterinary contexts. Identifying protective antigens in H. longicornis for an anti-tick vaccine is a key tick control strategy. Enolase, a multifunctional protein, significantly converts D-2-phosphoglycerate and phosphoenolpyruvate in glycolysis and gluconeogenesis in cell cytoplasm. This study cloned a complete open reading frame (ORF) of enolase from the H. longicornis tick and characterized its transcriptional and silencing effect. We amplified the full-length cDNA of the enolase gene using rapid amplification of cDNA ends. The complete cDNA, with an ORF of 1,297 nucleotides, encoded a 432-amino acid polypeptide. Enolase of the Jeju strain H. longicornis exhibited the highest sequence similarity with H. flava (98%), followed by Dermacentor silvarum (82%). The enolase motifs identified included N-terminal and C-terminal regions, magnesium binding sites, and several phosphorylation sites. Reverse transcription-polymerase chain reaction (RT-PCR) analysis indicated that enolase mRNA transcripts were expressed across all developmental stages of ticks and organs such as salivary gland and midgut. RT-PCR showed higher transcript levels in syn-ganglia, suggesting that synganglion nerves influence enolase,s role in tick salivary glands. We injected enolase double-stranded RNA into adult unfed female ticks, after which they were subsequently fed with normal unfed males until they spontaneously dropped off. RNA interference significantly (P<0.05) reduced feeding and reproduction, along with abnormalities in eggs (no embryos) and hatching. These findings suggest enolase is a promising target for future tick control strategies.
Keywords: Double-stranded RNA; RNA interference; knockdown; real-time PCR; transcript; vaccine.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

