Organ-specific Toxocara canis larvae migration and host immune response in experimentally infected mice
- PMID: 38835265
- PMCID: PMC11150926
- DOI: 10.3347/PHD.23125
Organ-specific Toxocara canis larvae migration and host immune response in experimentally infected mice
Abstract
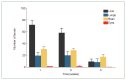
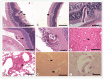
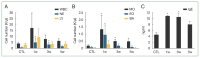
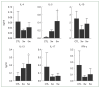
We investigated organ specific Toxocara canis larval migration in mice infected with T. canis larvae. We observed the worm burden and systemic immune responses. Three groups of BALB/c mice (n=5 each) were orally administered 1,000 T. canis 2nd stage larvae to induce larva migrans. Mice were sacrificed at 1, 3, and 5 weeks post-infection. Liver, lung, brain, and eye tissues were collected. Tissue from 2 mice per group was digested for larval count, while the remaining 3 mice underwent histological analysis. Blood hematology and serology were evaluated and compared to that in a control uninfected group (n=5) to assess the immune response. Cytokine levels in bronchoalveolar lavage (BAL) fluid were also analyzed. We found that, 1 week post-infection, the mean parasite load in the liver (72±7.1), brain (31±4.2), lungs (20±5.7), and eyes (2±0) peaked and stayed constant until the 3 weeks. By 5-week post-infection, the worm burden in the liver and lungs significantly decreased to 10±4.2 and 9±5.7, respectively, while they remained relatively stable in the brain and eyes (18±4.2 and 1±0, respectively). Interestingly, ocular larvae resided in all retinal layers, without notable inflammation in outer retina. Mice infected with T. canis exhibited elevated levels of neutrophils, monocytes, eosinophils, and immunoglobulin E. At 5 weeks post-infection, interleukin (IL)-5 and IL-13 levels were elevated in BAL fluid. Whereas IL-4, IL-10, IL-17, and interferon-γ levels in BAL fluid were similar to that in controls. Our findings demonstrate that a small portion of T. canis larvae migrate to the eyes and brain within the first week of infection. Minimal tissue inflammation was observed, probably due to increase of anti-inflammatory cytokines. This study contributes to our understanding of the histological and immunological responses to T. canis infection in mice, which may have implications to further understand human toxocariasis.
Keywords: Toxocara canis; host-parasite interaction; larva migrans; toxocariasis.
Conflict of interest statement
Figures
Similar articles
-
Persistent airway hyper-responsiveness and inflammation in Toxocara canis-infected BALB/c mice.Clin Exp Allergy. 2005 Jun;35(6):826-32. doi: 10.1111/j.1365-2222.2005.02250.x. Clin Exp Allergy. 2005. PMID: 15969676
-
Influence of murine Toxocara canis infection on plasma and bronchoalveolar lavage fluid eosinophil numbers and its correlation with cytokine levels.Vet Parasitol. 2005 Nov 25;134(1-2):121-30. doi: 10.1016/j.vetpar.2005.06.022. Epub 2005 Sep 15. Vet Parasitol. 2005. PMID: 16168564
-
Evidence for Asthma in the Lungs of Mice Inoculated with Different Doses of Toxocara canis.Am J Trop Med Hyg. 2020 Dec;103(6):2305-2314. doi: 10.4269/ajtmh.20-0484. Epub 2020 Sep 17. Am J Trop Med Hyg. 2020. PMID: 32975177 Free PMC article.
-
Pathogenesis of cerebral toxocariasis and neurodegenerative diseases.Adv Parasitol. 2020;109:233-259. doi: 10.1016/bs.apar.2020.01.008. Epub 2020 Feb 5. Adv Parasitol. 2020. PMID: 32381200 Review.
-
Role of sex hormones in the reactivation of Toxocara canis larvae in pregnant bitches.Vet Parasitol. 2025 Feb;334:110393. doi: 10.1016/j.vetpar.2025.110393. Epub 2025 Jan 9. Vet Parasitol. 2025. PMID: 39818124 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

