Emerging role of MYB transcription factors in cancer drug resistance
- PMID: 38835346
- PMCID: PMC11149108
- DOI: 10.20517/cdr.2023.158
Emerging role of MYB transcription factors in cancer drug resistance
Abstract
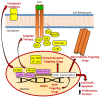
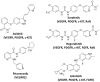
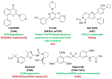
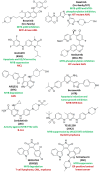
Decades ago, the viral myeloblastosis oncogene v-myb was identified as a gene responsible for the development of avian leukemia. However, the relevance of MYB proteins for human cancer diseases, in particular for solid tumors, remained basically unrecognized for a very long time. The human family of MYB transcription factors comprises MYB (c-MYB), MYBL2 (b-MYB), and MYBL1 (a-MYB), which are overexpressed in several cancers and are associated with cancer progression and resistance to anticancer drugs. In addition to overexpression, the presence of activated MYB-fusion proteins as tumor drivers was described in certain cancers. The identification of anticancer drug resistance mediated by MYB proteins and their underlying mechanisms are of great importance in understanding failures of current therapies and establishing new and more efficient therapy regimens. In addition, new drug candidates targeting MYB transcription factor activity and signaling have emerged as a promising class of potential anticancer therapeutics that could tackle MYB-dependent drug-resistant cancers in a more selective way. This review describes the correlation of MYB transcription factors with the formation and persistence of cancer resistance to various approved and investigational anticancer drugs.
Keywords: Anticancer drugs; MYB; MYB-targeting drugs; cancer resistance mechanisms; drug resistance; oncogenes; transcription factors.
© The Author(s) 2024.
Conflict of interest statement
Biersack B is a Junior Editorial Board Member of the journal Cancer Drug Resistance, while the other author has declared that he has no conflicts of interest.
Figures
Similar articles
-
From modulation of cellular plasticity to potentiation of therapeutic resistance: new and emerging roles of MYB transcription factors in human malignancies.Cancer Metastasis Rev. 2024 Mar;43(1):409-421. doi: 10.1007/s10555-023-10153-8. Epub 2023 Nov 10. Cancer Metastasis Rev. 2024. PMID: 37950087 Free PMC article. Review.
-
MYB oncoproteins: emerging players and potential therapeutic targets in human cancer.Oncogenesis. 2021 Feb 26;10(2):19. doi: 10.1038/s41389-021-00309-y. Oncogenesis. 2021. PMID: 33637673 Free PMC article. Review.
-
MYB, MYBL1, MYBL2 and NFIB gene alterations and MYC overexpression in salivary gland adenoid cystic carcinoma.Histopathology. 2017 Nov;71(5):823-834. doi: 10.1111/his.13281. Epub 2017 Aug 16. Histopathology. 2017. PMID: 28594149
-
The myb oncogene.Gene Amplif Anal. 1986;4:73-98. Gene Amplif Anal. 1986. PMID: 3333362 Review.
-
Modulation of c-Myb-induced transcription activation by a phosphorylation site near the negative regulatory domain.Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6429-33. doi: 10.1073/pnas.92.14.6429. Proc Natl Acad Sci U S A. 1995. PMID: 7604007 Free PMC article.
Cited by
-
Cynthichlorine Extracted from Ascidian Cynthia savignyi from Djibouti: Optimization of Extraction, In Vitro Anticancer Profiling, and In Silico Approach.Mar Drugs. 2025 Apr 16;23(4):172. doi: 10.3390/md23040172. Mar Drugs. 2025. PMID: 40278293 Free PMC article.
-
STAT3 Signaling Pathway in Health and Disease.MedComm (2020). 2025 Mar 30;6(4):e70152. doi: 10.1002/mco2.70152. eCollection 2025 Apr. MedComm (2020). 2025. PMID: 40166646 Free PMC article. Review.
-
Targeting histone acetylation to overcome drug resistance in the parasite Trichomonas vaginalis.bioRxiv [Preprint]. 2025 Jan 7:2025.01.07.631743. doi: 10.1101/2025.01.07.631743. bioRxiv. 2025. PMID: 39829914 Free PMC article. Preprint.
-
MYB Confers Sorafenib Resistance in Human Leukemia Cells via Inhibiting Ferroptosis Through FTH1 Upregulation.Genes (Basel). 2025 Jun 26;16(7):737. doi: 10.3390/genes16070737. Genes (Basel). 2025. PMID: 40725394 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
