A glutamatergic biomarker panel enables differentiating Grade 4 gliomas/astrocytomas from brain metastases
- PMID: 38835368
- PMCID: PMC11148222
- DOI: 10.3389/fonc.2024.1335401
A glutamatergic biomarker panel enables differentiating Grade 4 gliomas/astrocytomas from brain metastases
Abstract
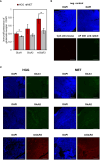
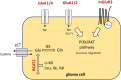
Background: The differentiation of high-grade glioma and brain tumors of an extracranial origin is eminent for the decision on subsequent treatment regimens. While in high-grade glioma, a surgical resection of the tumor mass is a fundamental part of current standard regimens, in brain metastasis, the burden of the primary tumor must be considered. However, without a cancer history, the differentiation remains challenging in the imaging. Hence, biopsies are common that may help to identify the tumor origin. An additional tool to support the differentiation may be of great help. For this purpose, we aimed to identify a biomarker panel based on the expression analysis of a small sample of tissue to support the pathological analysis of surgery resection specimens. Given that an aberrant glutamate signaling was identified to drive glioblastoma progression, we focused on glutamate receptors and key players of glutamate homeostasis.
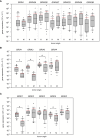
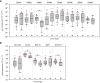
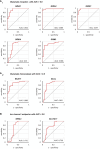
Methods: Based on surgically resected samples from 55 brain tumors, the expression of ionotropic and metabotropic glutamate receptors and key players of glutamate homeostasis were analyzed by RT-PCR. Subsequently, a receiver operating characteristic (ROC) analysis was performed to identify genes whose expression levels may be associated with either glioblastoma or brain metastasis.
Results: Out of a total of 29 glutamatergic genes analyzed, nine genes presented a significantly different expression level between high-grade gliomas and brain metastases. Of those, seven were identified as potential biomarker candidates including genes encoding for AMPA receptors GRIA1, GRIA2, kainate receptors GRIK1 and GRIK4, metabotropic receptor GRM3, transaminase BCAT1 and the glutamine synthetase (encoded by GLUL). Overall, the biomarker panel achieved an accuracy of 88% (95% CI: 87.1, 90.8) in predicting the tumor entity. Gene expression data, however, could not discriminate between patients with seizures from those without.
Conclusion: We have identified a panel of seven genes whose expression may serve as a biomarker panel to discriminate glioblastomas and brain metastases at the molecular level. After further validation, our biomarker signatures could be of great use in the decision making on subsequent treatment regimens after diagnosis.
Keywords: biomarker; brain metastasis; epilepsy; glioblastoma; glutamate; glutamate receptors.
Copyright © 2024 Lange, Gade, Einsle, Porath, Reichart, Maletzki, Schneider, Henker, Dubinski, Linnebacher, Köhling, Freiman and Kirschstein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Novel 3D magnetic resonance fingerprinting radiomics in adult brain tumors: a feasibility study.Eur Radiol. 2023 Feb;33(2):836-844. doi: 10.1007/s00330-022-09067-w. Epub 2022 Aug 24. Eur Radiol. 2023. PMID: 35999374
-
Glioblastoma cells induce differential glutamatergic gene expressions in human tumor-associated microglia/macrophages and monocyte-derived macrophages.Cancer Biol Ther. 2015;16(8):1205-13. doi: 10.1080/15384047.2015.1056406. Epub 2015 Jun 5. Cancer Biol Ther. 2015. PMID: 26047211 Free PMC article.
-
Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema.Acta Radiol. 2009 Jul;50(6):682-9. doi: 10.1080/02841850902933123. Acta Radiol. 2009. PMID: 19449234
-
Pathway analysis of glutamate-mediated, calcium-related signaling in glioma progression.Biochem Pharmacol. 2020 Jun;176:113814. doi: 10.1016/j.bcp.2020.113814. Epub 2020 Jan 16. Biochem Pharmacol. 2020. PMID: 31954716 Free PMC article. Review.
-
Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review.
Cited by
-
Identification and validation of LY6H and GRM3 as candidate biomarkers for Glioma-related epilepsy.Sci Rep. 2025 Apr 14;15(1):12833. doi: 10.1038/s41598-025-97333-4. Sci Rep. 2025. PMID: 40229486 Free PMC article.
References
-
- Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. . Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-oncology. (2020) 22:1073–113. doi: 10.1093/neuonc/noaa106 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

