A Klotho-Based Machine Learning Model for Prediction of both Kidney and Cardiovascular Outcomes in Chronic Kidney Disease
- PMID: 38835404
- PMCID: PMC11149992
- DOI: 10.1159/000538510
A Klotho-Based Machine Learning Model for Prediction of both Kidney and Cardiovascular Outcomes in Chronic Kidney Disease
Abstract
Introduction: This study aimed to develop and validate machine learning (ML) models based on serum Klotho for predicting end-stage kidney disease (ESKD) and cardiovascular disease (CVD) in patients with chronic kidney disease (CKD).
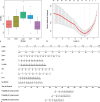
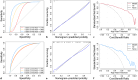
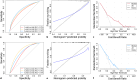
Methods: Five different ML models were trained to predict the risk of ESKD and CVD at three different time points (3, 5, and 8 years) using a cohort of 400 non-dialysis CKD patients. The dataset was divided into a training set (70%) and an internal validation set (30%). These models were informed by data comprising 47 clinical features, including serum Klotho. The best-performing model was selected and used to identify risk factors for each outcome. Model performance was assessed using various metrics.
Results: The findings showed that the least absolute shrinkage and selection operator regression model had the highest accuracy (C-index = 0.71) in predicting ESKD. The features mainly included in this model were estimated glomerular filtration rate, 24-h urinary microalbumin, serum albumin, phosphate, parathyroid hormone, and serum Klotho, which achieved the highest area under the curve (AUC) of 0.930 (95% CI: 0.897-0.962). In addition, for the CVD risk prediction, the random survival forest model with the highest accuracy (C-index = 0.66) was selected and achieved the highest AUC of 0.782 (95% CI: 0.633-0.930). The features mainly included in this model were age, history of primary hypertension, calcium, tumor necrosis factor-alpha, and serum Klotho.
Conclusion: We successfully developed and validated Klotho-based ML risk prediction models for CVD and ESKD in CKD patients with good performance, indicating their high clinical utility.
Keywords: Cardiovascular disease; Chronic kidney disease; End-stage kidney disease; Machine learning; Prediction model.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Machine learning model for cardiovascular disease prediction in patients with chronic kidney disease.Front Endocrinol (Lausanne). 2024 May 28;15:1390729. doi: 10.3389/fendo.2024.1390729. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38863928 Free PMC article.
-
ESKD Risk Prediction Model in a Multicenter Chronic Kidney Disease Cohort in China: A Derivation, Validation, and Comparison Study.J Clin Med. 2023 Feb 14;12(4):1504. doi: 10.3390/jcm12041504. J Clin Med. 2023. PMID: 36836039 Free PMC article.
-
Machine learning models to predict end-stage kidney disease in chronic kidney disease stage 4.BMC Nephrol. 2023 Dec 19;24(1):376. doi: 10.1186/s12882-023-03424-7. BMC Nephrol. 2023. PMID: 38114923 Free PMC article.
-
Adverse Pregnancy Outcomes and Long-term Maternal Kidney Disease: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Feb 5;3(2):e1920964. doi: 10.1001/jamanetworkopen.2019.20964. JAMA Netw Open. 2020. PMID: 32049292
-
Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease.Kidney Dis (Basel). 2017 Jul;3(1):15-23. doi: 10.1159/000452880. Epub 2016 Nov 17. Kidney Dis (Basel). 2017. PMID: 28785560 Free PMC article. Review.
Cited by
-
Predicting major adverse cardiac events in diabetes and chronic kidney disease: a machine learning study from the Silesia Diabetes-Heart Project.Cardiovasc Diabetol. 2025 Feb 15;24(1):76. doi: 10.1186/s12933-025-02615-w. Cardiovasc Diabetol. 2025. PMID: 39955553 Free PMC article.
References
-
- Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34(11):1803–5. - PubMed
-
- Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. . Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–22. - PubMed
-
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. . Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–100. - PubMed
LinkOut - more resources
Full Text Sources

