Nε-lysine acetylation of the histone-like protein HBsu influences antibiotic survival and persistence in Bacillus subtilis
- PMID: 38835483
- PMCID: PMC11148388
- DOI: 10.3389/fmicb.2024.1356733
Nε-lysine acetylation of the histone-like protein HBsu influences antibiotic survival and persistence in Bacillus subtilis
Abstract
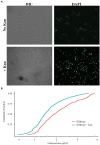
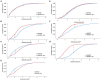
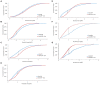
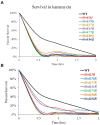
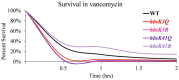
Nε-lysine acetylation is recognized as a prevalent post-translational modification (PTM) that regulates proteins across all three domains of life. In Bacillus subtilis, the histone-like protein HBsu is acetylated at seven sites, which regulates DNA compaction and the process of sporulation. In Mycobacteria, DNA compaction is a survival strategy in response antibiotic exposure. Acetylation of the HBsu ortholog HupB decondenses the chromosome to escape this drug-induced, non-growing state, and in addition, regulates the formation of drug-tolerant subpopulations by altering gene expression. We hypothesized that the acetylation of HBsu plays similar regulatory roles. First, we measured nucleoid area by fluorescence microscopy and in agreement, we found that wild-type cells compacted their nucleoids upon kanamycin exposure, but not exposure to tetracycline. We analyzed a collection of HBsu mutants that contain lysine substitutions that mimic the acetylated (glutamine) or unacetylated (arginine) forms of the protein. Our findings indicate that some level of acetylation is required at K3 for a proper response and K75 must be deacetylated. Next, we performed time-kill assays of wild-type and mutant strains in the presence of different antibiotics and found that interfering with HBsu acetylation led to faster killing rates. Finally, we examined the persistent subpopulation and found that altering the acetylation status of HBsu led to an increase in persister cell formation. In addition, we found that most of the deacetylation-mimic mutants, which have compacted nucleoids, were delayed in resuming growth following removal of the antibiotic, suggesting that acetylation is required to escape the persistent state. Together, this data adds an additional regulatory role for HBsu acetylation and further supports the existence of a histone-like code in bacteria.
Keywords: acetyl; acetylation; antibiotic resistance; bacteria; nucleoid-associated protein; persisters; tolerance.
Copyright © 2024 Carr, Tucker, Newman, Jadalla, Jaludi, Reid, Alpheaus, Korrapati, Pivonka and Carabetta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Nε-Lysine Acetylation of the Histone-Like Protein HBsu Regulates the Process of Sporulation and Affects the Resistance Properties of Bacillus subtilis Spores.Front Microbiol. 2022 Jan 17;12:782815. doi: 10.3389/fmicb.2021.782815. eCollection 2021. Front Microbiol. 2022. PMID: 35111139 Free PMC article.
-
YfmK is an Nε-lysine acetyltransferase that directly acetylates the histone-like protein HBsu in Bacillus subtilis.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3752-3757. doi: 10.1073/pnas.1815511116. Epub 2019 Feb 11. Proc Natl Acad Sci U S A. 2019. PMID: 30808761 Free PMC article.
-
Addressing the Possibility of a Histone-Like Code in Bacteria.J Proteome Res. 2021 Jan 1;20(1):27-37. doi: 10.1021/acs.jproteome.0c00442. Epub 2020 Oct 2. J Proteome Res. 2021. PMID: 32962352 Free PMC article. Review.
-
The Major Chromosome Condensation Factors Smc, HBsu, and Gyrase in Bacillus subtilis Operate via Strikingly Different Patterns of Motion.mSphere. 2020 Sep 9;5(5):e00817-20. doi: 10.1128/mSphere.00817-20. mSphere. 2020. PMID: 32907955 Free PMC article.
-
A novel mode of histone-like protein HupB regulating Sinorhizobium meliloti cell division through lysine acetylation.Curr Res Microb Sci. 2025 Jan 22;8:100345. doi: 10.1016/j.crmicr.2025.100345. eCollection 2025. Curr Res Microb Sci. 2025. PMID: 39926159 Free PMC article. Review.
Cited by
-
Important Role of Bacterial Nucleoid-Associated Proteins in Discovery of Novel Secondary Metabolites.Int J Mol Sci. 2025 Mar 7;26(6):2393. doi: 10.3390/ijms26062393. Int J Mol Sci. 2025. PMID: 40141036 Free PMC article. Review.
References
-
- Bigger J. (1944). Treatment of staphylococcal infections with penicillin by intermittent sterilization. Lancet 244, 497–500. doi: 10.1016/S0140-6736(00)74210-3 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

