Association of maternal postpartum depression symptoms with infant neurodevelopment and gut microbiota
- PMID: 38835546
- PMCID: PMC11148360
- DOI: 10.3389/fpsyt.2024.1385229
Association of maternal postpartum depression symptoms with infant neurodevelopment and gut microbiota
Abstract
Introduction: Understanding the mechanisms underlying maternal postpartum depression (PPD) and its effects on offspring development is crucial. However, research on the association between maternal PPD, gut microbiota, and offspring neurodevelopment remains limited. This study aimed to examine the association of maternal PPD symptoms with early gut microbiome, gut metabolome, and neurodevelopment in infants at 6 months.
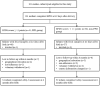
Methods: Maternal PPD symptoms were assessed using the Edinburgh Postpartum Depression Scale (EPDS) at 42 days postpartum. Infants stool samples collected at 42 days after birth were analyzed using 16S rRNA sequencing and liquid chromatography-mass spectrometry (LC-MS) detection. Infant neurodevelopment was measured at 6 months using the Ages and Stages Questionnaire, Third Edition (ASQ-3). Correlations between gut microbiota, metabolites and neurodevelopment were identified through co-occurrence network analysis. Finally, mediation analyses were conducted to determine potential causal pathways.
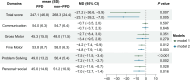
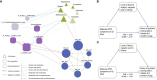
Results: A total of 101 mother-infant dyads were included in the final analysis. Infants born to mothers with PPD symptoms at 42 days postpartum had lower neurodevelopmental scores at 6 months. These infants also had increased alpha diversity of gut microbiota and were abundant in Veillonella and Finegoldia, while depleted abundance of Bifidobacterium, Dialister, Cronobacter and Megasphaera. Furthermore, alterations were observed in metabolite levels linked to the Alanine, aspartate, and glutamate metabolic pathway, primarily characterized by decreases in N-Acetyl-L-aspartic acid, L-Aspartic acid, and L-Asparagine. Co-occurrence network and mediation analyses revealed that N-Acetyl-L-aspartic acid and L-Aspartic acid levels mediated the relationship between maternal PPD symptoms and the development of infant problem-solving skills.
Conclusions: Maternal PPD symptoms are associated with alterations in the gut microbiota and neurodevelopment in infants. This study provides new insights into potential early intervention for infants whose mother experienced PPD. Further research is warranted to elucidate the biological mechanisms underlying these associations.
Keywords: gut metabolome; gut microbiome; gut-brain axis; neurodevelopment; postpartum depression.
Copyright © 2024 Zhou, Tang, Zhou, Wen, Krewski and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer ZH declared a shared affiliation with the authors SW, DK to the handling editor at the time of review.
Figures

References
LinkOut - more resources
Full Text Sources

