Efficacy and Safety of Infliximab Versus Adalimumab in Adult Subjects With Moderate to Severe Ulcerative Colitis: A Systematic Review and Meta-Analysis
- PMID: 38835557
- PMCID: PMC11148671
- DOI: 10.7759/cureus.61547
Efficacy and Safety of Infliximab Versus Adalimumab in Adult Subjects With Moderate to Severe Ulcerative Colitis: A Systematic Review and Meta-Analysis
Abstract
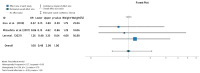
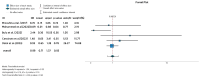
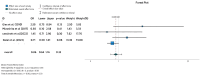
Ulcerative colitis (UC) is an inflammatory disorder affecting the colon, and typically, during the disease course, the condition may exacerbate, relapse, and remit. One of the most successful lines for inducing and maintaining clinical remission in subjects with UC is biological therapy with anti-tumor necrosis factor α (anti-TNF) agents, including adalimumab (ADA) and infliximab (IFX). This meta-analysis is an attempt to obtain complementary information driven by real-world experience (RWE) concerning the efficacy and safety of two of the most popular anti-TNFs in treating UC. This is a systematic review and meta-analysis of RWE studies comparing ADA and IFX as naïve anti-TNF agents for the treatment of subjects with UC. Studies were obtained by searching Scopus, Google Scholar, the Cochrane Central Register of Controlled Trials, Embase, and the PubMed Central databases. Patients treated with IFX showed significantly higher induction responses. No statistically significant difference was found in the comparison of response in the maintenance treatment period. Higher overall adverse events were related to IFX treatment, with serious adverse events that were nonsignificantly higher in the ADA-treated group. In conclusion, IFX demonstrated significantly higher induction responses compared to ADA in patients with moderate-to-severe UC. IFX was associated with higher overall adverse events, whereas serious adverse events were non-significantly higher in the ADA-treated group. IFX may be favored as a first-line agent for its induction efficacy, and the choice between IFX and ADA should be individualized based on comprehensive clinical evaluation.
Keywords: adalimumab; infliximab; meta-analysis; real-world experience; ulcerative colitis.
Copyright © 2024, Salman et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





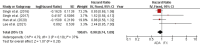
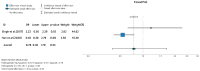

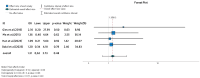


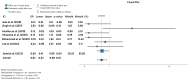
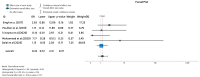

Similar articles
-
Infliximab Originator, Infliximab Biosimilar, and Adalimumab Are More Effective in Crohn's Disease Than Ulcerative Colitis: A Real-Life Cohort Study.Clin Transl Gastroenterol. 2020 May;11(5):e00177. doi: 10.14309/ctg.0000000000000177. Clin Transl Gastroenterol. 2020. PMID: 32677808 Free PMC article.
-
Comparative effectiveness and safety of infliximab and adalimumab in patients with ulcerative colitis.Aliment Pharmacol Ther. 2016 May;43(9):994-1003. doi: 10.1111/apt.13580. Epub 2016 Mar 15. Aliment Pharmacol Ther. 2016. PMID: 26991059 Free PMC article.
-
Anti-TNF therapy for ulcerative colitis in Brazil: a comparative real-world national retrospective multicentric study from the Brazilian study group of IBD (GEDIIB).BMC Gastroenterol. 2022 May 29;22(1):268. doi: 10.1186/s12876-022-02341-7. BMC Gastroenterol. 2022. PMID: 35644668 Free PMC article.
-
Efficacy and Safety of Adalimumab in Moderately to Severely Active Cases of Ulcerative Colitis: A Meta-Analysis of Published Placebo-Controlled Trials.Gut Liver. 2016 Mar;10(2):262-74. doi: 10.5009/gnl15042. Gut Liver. 2016. PMID: 26780088 Free PMC article. Review.
-
Anti-tumor necrosis factor agents in sarcoidosis: A systematic review of efficacy and safety.Semin Arthritis Rheum. 2019 Jun;48(6):1093-1104. doi: 10.1016/j.semarthrit.2018.10.005. Epub 2018 Oct 16. Semin Arthritis Rheum. 2019. PMID: 30446173
Cited by
-
Efficacy and safety of tanshinone IIA in combination with mesalazine in the treatment of ulcerative colitis: a Systematic review and meta-analysis.BMC Gastroenterol. 2024 Nov 15;24(1):410. doi: 10.1186/s12876-024-03496-1. BMC Gastroenterol. 2024. PMID: 39548391 Free PMC article.
References
-
- Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Feagan BG, Sandborn WJ, Lazar A, et al. Gastroenterology. 2014;146:110–118. - PubMed
-
- Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Danese S, Fiorino G, Peyrin-Biroulet L, Lucenteforte E, Virgili G, Moja L, Bonovas S. Ann Intern Med. 2014;160:704–711. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
