Meta-analysis of the effects of CYP3A5*3 gene polymorphisms on tacrolimus blood concentration and effectiveness in Chinese patients with membranous nephropathy
- PMID: 38835664
- PMCID: PMC11148365
- DOI: 10.3389/fphar.2024.1385322
Meta-analysis of the effects of CYP3A5*3 gene polymorphisms on tacrolimus blood concentration and effectiveness in Chinese patients with membranous nephropathy
Abstract
Objective: The study aimed to systematically evaluate the relationship between CYP3A5*3 gene polymorphisms and the blood concentration and effectiveness of tacrolimus (TAC) in patients with membranous nephropathy (MN).
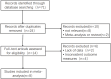
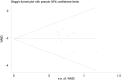
Methods: PubMed, Cochrane Library, Embase, Web of Science, China Biomedical, China National Knowledge Infrastructure, Wanfang, Vipshop, ReadShow, Clinical Trials Registry, and other databases were searched. Studies on the relationship between CYP3A5*3 gene polymorphism and TAC blood concentration in MN patients were collected, and meta-analysis was performed using Stata 16 software.
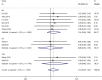
Results: A total of eight publications were included in the study, including 498 MN patients. CYP3A5*3 gene polymorphisms are associated with tacrolimus blood levels in patients with MN. The results of the relationship between CYP3A5*3 genotype polymorphisms and tacrolimus blood trough concentrations of the AA + AG genotype were lower than those of the GG genotype at ≤1 month [WMD = -2.08, 95% CI (-2.57, -1.59), p < 0.001] and 1-6 months [WMD = -0.63, 95% CI (-0.98, -0.27), p < 0.001]; however, they were not statistically significant at ≥6 months (p = 0.211). Furthermore, the subgroup analysis revealed that the dose-adjusted concentration of tacrolimus (C0/D) of the AA + AG genotype was lower than that of the GG genotype at ≤1 month [SMD = -1.93, 95% CI (-2.79, -1.08), p < 0.001], 1-6 months [SMD = -2.25, 95% CI (-2.71, -1.79), p < 0.001], and ≥6 months [SMD = -2.36, 95% CI (-2.86, -1.86), p < 0.001]. In addition, there was no statistically significant difference in effectiveness between the two groups at 3, 6, and 12 months of TAC administration (p > 0.05).
Conclusion: Serum TAC concentrations in MN patients were correlated with CYP3A5*3 genotype polymorphisms. Detection of the CYP3A5*3 genotype before the administration of TAC may provide some clinical value for optimizing the treatment of MN patients.
Systematic review registration: https://inplasy.com/, identifier [INPLASY202430083].
Keywords: CYP3A5; gene polymorphism; membranous nephropathy; meta-analysis; tacrolimus.
Copyright © 2024 Dai, Yuan and Chai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

