Investigating Sulforaphane's anti-virulence and anti-quorum sensing properties against Pseudomonas aeruginosa
- PMID: 38835668
- PMCID: PMC11148281
- DOI: 10.3389/fphar.2024.1406653
Investigating Sulforaphane's anti-virulence and anti-quorum sensing properties against Pseudomonas aeruginosa
Abstract
Background: P. aeruginosa, a significant bacterium, can cause severe illness and resistance to antibiotics. Quorum sensing (QS) systems regulate virulence factors production. Targeting QS could reduce bacteria pathogenicity and prevent antibiotic resistance. Cruciferous vegetables contain sulforaphane, known for its anti-inflammatory, antioxidant, anticancer, and antimicrobial properties.
Aim: We aimed to examine the inhibitory influences of sulforaphane, at a sub-inhibitory concentration (¼ minimum inhibitory concentration, MIC), on virulence and QS in P. aeruginosa.
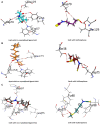
Materials and methods: The sulforaphane's anti-virulence actions at sub-inhibitory concentrations were explored in vitro and in vivo. A sub-MIC concentration of sulforaphane was combined with anti-pseudomonal drugs, and the results of this combination were assessed. The virtual affinity of sulforaphane for the receptors of QS was studied, and its effect on the expression of QS genes was quantified.
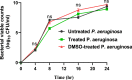
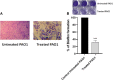
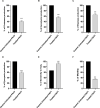
Results: Sulforaphane significantly decreased the biofilm formation, motility, ability to withstand oxidative stress, and the synthesis of virulence extracellular enzymes such as proteases, hemolysins, and elastase, as well as other virulence factors like pyocyanin. In addition, sulforaphane lessened the severity of P. aeruginosa infection in mice. Sulforaphane reduced the antipseudomonal antibiotics' MICs when used together, resulting in synergistic effects. The observed anti-virulence impacts were attributed to the ability of sulforaphane to inhibit QS via suppressing the QS genes' expression.
Conclusion: Sulforaphane shows promise as a potent anti-virulence and anti-QS agent that can be used alongside conventional antimicrobials to manage severe infections effectively. Furthermore, this study paves the way for further investigation of sulforaphane and similar structures as pharmacophores for anti-QS candidates.
Keywords: Pseudomonas aeruginosa; Sulforaphane; bacterial virulence; quorum sensing; resistance to antibiotics.
Copyright © 2024 Bendary, Ali, Abdel Halim, Boufahja, Chaudhary, Elkelish, Soliman and Hegazy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model.PLoS One. 2017 Apr 28;12(4):e0176883. doi: 10.1371/journal.pone.0176883. eCollection 2017. PLoS One. 2017. PMID: 28453568 Free PMC article.
-
Exploration of the inhibitory effect of Cassia fistula on quorum sensing mediated virulence factor production and biofilm activity in Pseudomonas aeruginosa: an in vivo study in model organism Caenorhabditis elegans.J Med Microbiol. 2023 Feb;72(2). doi: 10.1099/jmm.0.001578. J Med Microbiol. 2023. PMID: 36787160
-
Antimicrobial and anti-virulence activities of 4-shogaol from grains of paradise against gram-negative bacteria: Integration of experimental and computational methods.J Ethnopharmacol. 2024 Apr 6;323:117611. doi: 10.1016/j.jep.2023.117611. Epub 2023 Dec 27. J Ethnopharmacol. 2024. PMID: 38158095
-
Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors.Microb Pathog. 2021 Jun;155:104912. doi: 10.1016/j.micpath.2021.104912. Epub 2021 Apr 28. Microb Pathog. 2021. PMID: 33932548 Review.
-
Repurposing phytochemicals as anti-virulent agents to attenuate quorum sensing-regulated virulence factors and biofilm formation in Pseudomonas aeruginosa.Microb Biotechnol. 2022 Jun;15(6):1695-1718. doi: 10.1111/1751-7915.13981. Epub 2021 Nov 29. Microb Biotechnol. 2022. PMID: 34843159 Free PMC article. Review.
Cited by
-
Beyond defense: microbial modifications of plant specialized metabolites alter and expand their ecological functions.New Phytol. 2025 Jul;247(2):518-526. doi: 10.1111/nph.70254. Epub 2025 May 30. New Phytol. 2025. PMID: 40444642 Free PMC article. Review.
-
Sulforaphane Is Not Only a Food Supplement: It Diminishes the Intracellular Survival and Colonization of Salmonella enterica.ACS Omega. 2025 Jan 16;10(3):2969-2977. doi: 10.1021/acsomega.4c09408. eCollection 2025 Jan 28. ACS Omega. 2025. PMID: 39895767 Free PMC article.
-
Reprofiling lamivudine as an antibiofilm and anti-pathogenic agent against Pseudomonas aeruginosa.AMB Express. 2025 Feb 22;15(1):33. doi: 10.1186/s13568-025-01835-3. AMB Express. 2025. PMID: 39985628 Free PMC article.
-
Antibiotic-Resistant Pseudomonas aeruginosa: Current Challenges and Emerging Alternative Therapies.Microorganisms. 2025 Apr 16;13(4):913. doi: 10.3390/microorganisms13040913. Microorganisms. 2025. PMID: 40284749 Free PMC article. Review.
-
Sorbate metal complexes as newer antibacterial, antibiofilm, and anticancer compounds.BMC Microbiol. 2024 Jul 18;24(1):262. doi: 10.1186/s12866-024-03370-w. BMC Microbiol. 2024. PMID: 39026170 Free PMC article.
References
-
- Abu Lila A. S., Alharby T. N., Alanazi J., Alanazi M., Abdallah M. H., Rizvi S. M. D., et al. (2023). Clinical resistant strains of enterococci and their correlation to reduced susceptibility to biocides: phenotypic and genotypic analysis of macrolides, lincosamides, and streptogramins. Antibiotics 12, 461. 10.3390/antibiotics12030461 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

