Tumor spheroids and organoids as preclinical model systems
- PMID: 38835698
- PMCID: PMC11006296
- DOI: 10.1515/medgen-2021-2093
Tumor spheroids and organoids as preclinical model systems
Abstract
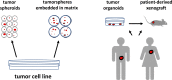
The generation of three-dimensional (3D) cancer models is a novel and fascinating development in the study of personalized medicine and tumor-specific drug delivery. In addition to the classical two-dimensional (2D) adherent cell culture models, 3D spheroid and organoid cancer models that mimic the microenvironment of cancer tissue are emerging as an important preclinical model system. 3D cancer models form, similar to cancer, multiple cell-cell and cell-extracellular matrix interactions and activate different cellular cascades/pathways, like proliferation, quiescence, senescence, and necrotic or apoptotic cell death. Further, it is possible to analyze genetic variations and mutations, the microenvironment of cell-cell interactions, and the uptake of therapeutics and nanoparticles in nanomedicine. Important is also the analysis of cancer stem cells (CSCs), which could play key roles in resistance to therapy and cancer recurrence. Tumor spheroids can be generated from one tumor-derived cell line or from co-culture of two or more cell lines. Tumor organoids can be derived from tumors or may be generated from CSCs that differentiate into multiple facets of cancerous tissue. Similarly, tumorspheres can be generated from a single CSC. By transplanting spheroids and organoids into immune-deficient mice, patient-derived xenografts can serve as a preclinical model to test therapeutics in vivo. Although the handling and analysis of 3D tumor spheroids and organoids is more complex, it will provide insights into various cancer processes that cannot be provided by 2D culture. Here a short overview of 3D tumor systems as preclinical models is provided.
Keywords: Cancer organoids; patient-dervied xenograft; three-dimensional cancer model; tumor model systems.
© 2021 Baniahmad, published by De Gruyter.
Conflict of interest statement
Competing interests: The author states no conflict of interest.
Figures

References
-
- Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–96. - PubMed
-
- Gorgoulis. et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179(4):813–27. - PubMed
-
- Collado M. et al. Tumour biology: senescence in premalignant tumours. Nature. 2005 NaN;436(7051):642. - PubMed
LinkOut - more resources
Full Text Sources
