Developing a PmSLP3-based vaccine formulation that provides robust long-lasting protection against hemorrhagic septicemia-causing serogroup B and E strains of Pasteurella multocida in cattle
- PMID: 38835751
- PMCID: PMC11148319
- DOI: 10.3389/fimmu.2024.1392681
Developing a PmSLP3-based vaccine formulation that provides robust long-lasting protection against hemorrhagic septicemia-causing serogroup B and E strains of Pasteurella multocida in cattle
Abstract
Background: Pasteurella multocida is a bacterial pathogen that causes a variety of infections across diverse animal species, with one of the most devastating associated diseases being hemorrhagic septicemia. Outbreaks of hemorrhagic septicemia in cattle and buffaloes are marked by rapid progression and high mortality. These infections have particularly harmful socio-economic impacts on small holder farmers in Africa and Asia who are heavily reliant on a small number of animals kept as a means of subsistence for milk and draft power purposes. A novel vaccine target, PmSLP-3, has been identified on the surface of hemorrhagic septicemia-associated strains of P. multocida and was previously shown to elicit robust protection in cattle against lethal challenge with a serogroup B strain.
Methods: Here, we further investigate the protective efficacy of this surface lipoprotein, including evaluating the immunogenicity and protection upon formulation with a variety of adjuvants in both mice and cattle.
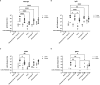
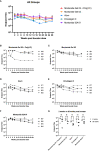
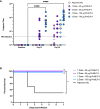
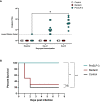
Results: PmSLP-3 formulated with Montanide ISA 61 elicited the highest level of serum and mucosal IgG, elicited long-lasting serum antibodies, and was fully protective against serogroup B challenge. Studies were then performed to identify the minimum number of doses required and the needed protein quantity to maintain protection. Duration studies were performed in cattle, demonstrating sustained serum IgG titres for 3 years after two doses of vaccine and full protection against lethal serogroup B challenge at 7 months after a single vaccine dose. Finally, a serogroup E challenge study was performed, demonstrating that PmSLP-3 vaccine can provide protection against challenge by the two serogroups responsible for hemorrhagic septicemia.
Conclusion: Together, these data indicate that PmSLP-3 formulated with Montanide ISA 61 is an immunogenic and protective vaccine against hemorrhagic septicemia-causing P. multocida strains in cattle.
Keywords: Pasteurella multocida; gram-negative; hemorrhagic septicemia; surface lipoprotein; vaccine.
Copyright © 2024 Fegan, Waeckerlin, Tesfaw, Islam, Deresse, Dufera, Assefa, Woldemedhin, Legesse, Akalu, Bayissa, Nguyen, Ng, Ahn, Schryvers, Tefera, Moraes and Gray-Owen.
Conflict of interest statement
TM, AS, and SG-O are co-authors on a patent, “Slam polynucleotides and polypeptides and uses thereof” - Patent Number: WO2017136947A1. EI, JF, AS, SG-O, and TM are coauthors on a provisional patent, “Veterinary vaccines and methods for the treatment of Pasteurella multocida infections in food production animals” - United States Provisional Application No. 63/332,966 or WO/2023/201434 and PCT/CA2023/050537.
Figures




References
-
- Carter GR. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. (1955) 16:481–4. - PubMed
-
- Rohkohl J, Schulze C, Bilk S. Hemorrhagic septicemia on a dairy farm in Lower Saxony. Der Praktische Tierarzt. (2015) 96:598–608.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

