Sex-biased immunogenicity of a mucosal subunit vaccine against SARS-CoV-2 in mice
- PMID: 38835757
- PMCID: PMC11148259
- DOI: 10.3389/fimmu.2024.1386243
Sex-biased immunogenicity of a mucosal subunit vaccine against SARS-CoV-2 in mice
Abstract
Introduction: Current vaccines against COVID-19 administered via parenteral route have limited ability to induce mucosal immunity. There is a need for an effective mucosal vaccine to combat SARS-CoV-2 virus replication in the respiratory mucosa. Moreover, sex differences are known to affect systemic antibody responses against vaccines. However, their role in mucosal cellular responses against a vaccine remains unclear and is underappreciated.
Methods: We evaluated the mucosal immunogenicity of a booster vaccine regimen that is recombinant protein-based and administered intranasally in mice to explore sex differences in mucosal humoral and cellular responses.
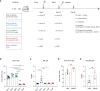
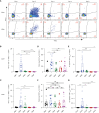
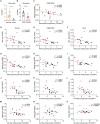
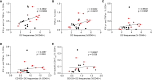
Results: Our results showed that vaccinated mice elicited strong systemic antibody (Ab), nasal, and bronchiole alveolar lavage (BAL) IgA responses, and local T cell immune responses in the lung in a sex-biased manner irrespective of mouse genetic background. Monocytes, alveolar macrophages, and CD103+ resident dendritic cells (DCs) in the lungs are correlated with robust mucosal Ab and T cell responses induced by the mucosal vaccine.
Discussion: Our findings provide novel insights into optimizing next-generation booster vaccines against SARS-CoV-2 by inducing spike-specific lung T cell responses, as well as optimizing mucosal immunity for other respiratory infections, and a rationale for considering sex differences in future vaccine research and vaccination practice.
Keywords: SARS-CoV-2; and sex differences; innate immunity; lung tissue-resident T cells; mucosal vaccine.
Copyright © 2024 Li, Hsu, Howe, Hoang, Xia, Berzofsky and Sui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Gunale B, Kapse D, Kar S, Bavdekar A, Kohli S, Lalwani S, et al. . Safety and immunogenicity of Sars-Cov-2 recombinant spike protein vaccine in children and adolescents in India: A phase 2–3 randomized clinical trial. JAMA Pediatr. (2023) 177:911–20. doi: 10.1001/jamapediatrics.2023.2552 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

