Efferocytosis in dendritic cells: an overlooked immunoregulatory process
- PMID: 38835772
- PMCID: PMC11148234
- DOI: 10.3389/fimmu.2024.1415573
Efferocytosis in dendritic cells: an overlooked immunoregulatory process
Abstract
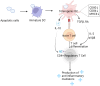
Efferocytosis, the process of engulfing and removing apoptotic cells, plays an essential role in preserving tissue health and averting undue inflammation. While macrophages are primarily known for this task, dendritic cells (DCs) also play a significant role. This review delves into the unique contributions of various DC subsets to efferocytosis, highlighting the distinctions in how DCs and macrophages recognize and handle apoptotic cells. It further explores how efferocytosis influences DC maturation, thereby affecting immune tolerance. This underscores the pivotal role of DCs in orchestrating immune responses and sustaining immune equilibrium, providing new insights into their function in immune regulation.
Keywords: Treg differentiation; antigen cross-presentation; apoptotic cell; dendritic cell; efferocytosis; immune regulation.
Copyright © 2024 Ma, Jiang, Zhu, Xu, Wan, Zhang and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

