Association between 23 drugs and inflammatory bowel disease: a two-sample Mendelian randomization study
- PMID: 38835788
- PMCID: PMC11149542
- DOI: 10.3389/fmed.2024.1371362
Association between 23 drugs and inflammatory bowel disease: a two-sample Mendelian randomization study
Abstract
Background: Inflammatory bowel disease (IBD) is a group of diseases characterized by chronic and recurrent inflammation of the gastrointestinal tract. The etiology of IBD remains multifaceted and poorly understood, resulting in limited treatment options that primarily target disease induction and remission maintenance. Thus, the exploration of novel therapeutic options for IBD among existing medications is advantageous. Mendelian randomization analysis (MR) serves as a valuable tool in investigating the relationship between drugs and diseases. In this study, MR analysis was employed to investigate the potential causal relationship between 23 approved drugs for the treatment of various diseases and IBD.
Method: We performed a two-sample MR analysis using publicly available genome-wide association study (GWAS) statistics. The inverse variance weighting (IVW) method was used as the main analysis method, supplemented by the remaining four methods (weighted median, MR Egger regression, simple and weighted models), and Meta-analysis was performed to expand the sample size to obtain a more reliable composite causal effect. Finally, Cochran's Q statistic and the MR-Egger test for directed pleiotropy were applied to determine whether significant heterogeneity or directed pleiotropy existed.
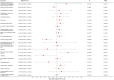
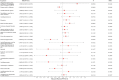
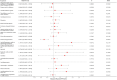
Results: In the main MR analysis (IVW), drugs with a negative causal association with the risk of IBD were immunosuppressant {OR (95% CI) = 0.7389 [0.6311-0.8651], p = 0.0046} and diabetes drugs {OR (95% CI) = 0.9266 [0.8876-0.9674], p = 0.0058}. A positive causal association with the risk of IBD was found for salicylic acid and derivatives {OR (95% CI) = 1.2737 [1.0778-1.5053], p = 0.0345}. Negative causal associations with UC risk were identified for immunosuppressants {OR (95% CI) = 0.6660 [0.5133-0.8640], p = 0.0169} and diabetes medications {OR (95% CI) = 0.9020 [0.8508-0.9551], p = 0.0046}; positive causal associations with UC risk were found for β-receptor blockers {OR (95% CI) = 1.1893 [1.0823-1.3070], p = 0.0046}. A negative causal association with the risk of CD was found for immunosuppressants {OR (95% CI) = 0.6957 [0.5803-0.8341], p = 0.0023}. There was no statistically significant association between the remaining 19 drugs and IBD and subtypes.
Conclusion: This MR study provides evidence suggesting that immunosuppressants have a mitigating effect on the risk of IBD and demonstrate consistent efficacy in subtypes of ulcerative colitis (UC) and Crohn's disease (CD). Additionally, diabetes medications show potential in reducing the risk of IBD, particularly in cases of UC, while β-blockers may elevate the risk of UC. Conversely, salicylic acid and its derivatives may increase the risk of IBD, although this effect is not consistently observed in the subtypes of the disease. These findings offer new insights into the prevention and management of IBD.
Keywords: 23 drugs; Crohn’s disease; Mendelian randomization; inflammatory bowel disease; ulcerative colitis.
Copyright © 2024 He, Deng, Huang, Yang, Yang and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Causal association between inflammatory bowel disease and IgA nephropathy: A bidirectional two-sample Mendelian randomization study.Front Genet. 2022 Nov 16;13:1002928. doi: 10.3389/fgene.2022.1002928. eCollection 2022. Front Genet. 2022. PMID: 36467999 Free PMC article.
-
Relationship between inflammatory bowel disease and erectile dysfunction: a 2-sample Mendelian randomization study.Sex Med. 2024 Jan 23;11(6):qfad067. doi: 10.1093/sexmed/qfad067. eCollection 2023 Dec. Sex Med. 2024. PMID: 38264202 Free PMC article.
-
Exploring the causal relationship between Takayasu arteritis and inflammatory bowel disease using Mendelian randomization.Immunol Res. 2024 Aug;72(4):707-713. doi: 10.1007/s12026-024-09476-7. Epub 2024 Mar 27. Immunol Res. 2024. PMID: 38536561
-
Exploring the causal relationship between interleukin-6 or C reactive protein and malignant melanoma using a two-sample Mendelian randomization approach.Front Oncol. 2024 Jun 17;14:1375362. doi: 10.3389/fonc.2024.1375362. eCollection 2024. Front Oncol. 2024. PMID: 38952546 Free PMC article. Review.
-
The Role of Topical Tacrolimus in the Management of Inflammatory Bowel Disease: A Comprehensive Review.J Clin Med. 2024 Sep 18;13(18):5518. doi: 10.3390/jcm13185518. J Clin Med. 2024. PMID: 39337004 Free PMC article. Review.
Cited by
-
The Association Between Fast Food Consumption and Inflammatory Bowel Disease: A Case-Control Study and Meta-Analysis.Nutrients. 2025 May 28;17(11):1838. doi: 10.3390/nu17111838. Nutrients. 2025. PMID: 40507107 Free PMC article.
References
-
- Fabián O, Kamaradová K. Morphology of inflammatory bowel diseases (IBD). Cesk Patol. (2022) 58:27–37. - PubMed
-
- Chen X, Xiang X, Xia W, Li X, Wang S, Ye S, et al. . Evolving trends and burden of inflammatory bowel disease in Asia, 1990-2019: a comprehensive analysis based on the global burden of disease study. J Epidemiol Glob Health. (2023) 13:725–39. doi: 10.1007/s44197-023-00145-w, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

