Evaluation of major adverse events of clozapine based on accordance to an international titration guideline
- PMID: 38835819
- PMCID: PMC11147652
- DOI: 10.9740/mhc.2024.06.204
Evaluation of major adverse events of clozapine based on accordance to an international titration guideline
Abstract
Introduction: Clozapine is the only antipsychotic approved for treatment-resistant schizophrenia, but without appropriate monitoring, it can be associated with potentially fatal outcomes. An International Adult Clozapine Titration Guideline categorizes patients into normal or slow metabolizers. Categorization provides clozapine titration schedules and recommends regular c-reactive protein (CRP) and clozapine concentration monitoring to reduce the risk of adverse drug reactions (ADRs). The impact of the guideline on clozapine ADRs has not been evaluated.
Methods: A retrospective chart review assessed clozapine titrations, laboratory monitoring, ADRs, and discontinuations for clozapine-naive adult inpatients at a single center from January 1, 2013, to June 1, 2022. Each patient's cumulative weekly clozapine dosage was compared with their guideline recommended dosage to create a percent accordance. Linear logistic regression evaluated the relationship between titration speed and the presence of an ADR, while descriptive statistics analyzed laboratory monitoring.
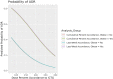
Results: Forty-three patients were included, with the majority being White males with schizophrenia. An inverse relationship existed between the last inpatient week clozapine dose percent accordance and the probability of an ADR. Nonobese patients were less likely than obese patients to experience an ADR (odds ratio = 0.17; 95% CI, 0.03-0.99). CRP and clozapine concentration monitoring was suboptimal.
Discussion: Based on our small retrospective review of primarily White males, more aggressive clozapine titrations did not increase ADRs. Future studies with more diverse samples are needed and should focus on specific ADRs, which may have increased occurrence with rapid titrations. Obese patients were at higher risk of ADRs, correlating with the guideline-recommended slower titrations for these patients.
Keywords: antipsychotic agents; clozapine; dose-response relationship; drug; drug-related side effects and adverse reactions; inflammation; schizophrenia spectrum and other psychotic disorders.
© 2024 AAPP. The Mental Health Clinician is a publication of the American Association of Psychiatric Pharmacists.
Figures

Similar articles
-
An International Adult Guideline for Making Clozapine Titration Safer by Using Six Ancestry-Based Personalized Dosing Titrations, CRP, and Clozapine Levels.Pharmacopsychiatry. 2022 Mar;55(2):73-86. doi: 10.1055/a-1625-6388. Epub 2021 Dec 15. Pharmacopsychiatry. 2022. PMID: 34911124
-
Pharmacovigilance in Action: Utilizing VigiBase Data to Improve Clozapine Safety.Patient Prefer Adherence. 2024 Nov 12;18:2261-2280. doi: 10.2147/PPA.S495254. eCollection 2024. Patient Prefer Adherence. 2024. PMID: 39553897 Free PMC article. Review.
-
An international guideline with six personalised titration schedules for preventing myocarditis and pneumonia associated with clozapine.Gen Psychiatr. 2022 Jun 29;35(3):e100773. doi: 10.1136/gpsych-2022-100773. eCollection 2022. Gen Psychiatr. 2022. PMID: 35866000 Free PMC article.
-
Exploring the potential effect of polypharmacy on the hematologic profiles of clozapine patients.J Psychiatr Pract. 2014 Jan;20(1):50-8. doi: 10.1097/01.pra.0000442937.61575.26. J Psychiatr Pract. 2014. PMID: 24419309 Review.
-
Should we routinely add CRP to clozapine titrations? - Learning from three cases.Neuropsychopharmacol Hung. 2022 Dec 1;24(4):153-161. Neuropsychopharmacol Hung. 2022. PMID: 36775960
Cited by
-
High frequency of clozapine-associated myocarditis and troponin elevation: Need for slower titration prospective studies.Ment Health Clin. 2024 Oct 1;14(5):302-303. doi: 10.9740/mhc.2024.10.302. eCollection 2024 Oct. Ment Health Clin. 2024. PMID: 39371482 Free PMC article. No abstract available.
References
-
- Accord Healthcare Inc. Bethesda (MD): National Library of Medicine (US) ; [updated 2023 Jan; cited 2023 Feb 23]. Clozapine tablet; [about 2 screens]. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=25c0c6d5-f7b0-4...
-
- Kikuchi Y, Komatsu H, Otsuka Y, Ito F, Kanahara N, Tanifuji H, et al. Slower clozapine titration than the official Japanese protocol led to fewer inflammatory adverse effects: A retrospective chart review of seven hospitals. Schizophr Res. Online ahead of print June 16, 2023. 10.1016/j.schres.2023.06.003 - DOI - PubMed
-
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. 10.1111/bdi.12609 - DOI - PMC - PubMed
-
- Yunusa I, Rashid N, Seyedin R, Paratane D, Rajagopalan K. Comparative efficacy, safety, and acceptability of pimavanserin and other atypical antipsychotics for Parkinson’s disease psychosis: Systematic review and network meta-analysis. J Geriatr Psychiatry Neurol. 2023:8919887231154933. 10.1177/08919887231154933 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
