Developmental and epileptic encephalopathies - therapeutic consequences of genetic testing
- PMID: 38835873
- PMCID: PMC11006352
- DOI: 10.1515/medgen-2022-2145
Developmental and epileptic encephalopathies - therapeutic consequences of genetic testing
Abstract
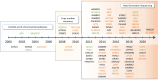
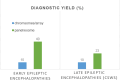
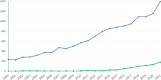
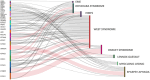
Developmental and epileptic encephalopathies comprise a heterogeneous group of monogenic neurodevelopmental disorders characterized by early-onset seizures, marked epileptic activity and abnormal neurocognitive development. The identification of an increasing number of underlying genetic alterations and their pathophysiological roles in cellular signaling drives the way toward novel precision therapies. The implementation of novel treatments that target the underlying mechanisms gives hope for disease modification that will improve not only the seizure burden but also the neurodevelopmental outcome of affected children. So far, beneficial effects are mostly reported in individual trials and small numbers of patients. There is a need for international collaborative studies to define the natural history and relevant outcome measures and to test novel pharmacological approaches.
Keywords: children; developmental and epileptic encephalopathy; monogenic etiology; precision treatment.
© 2022 the author(s), published by De Gruyter.
Conflict of interest statement
Competing interests: Authors state no conflict of interest.
Figures
Similar articles
-
Adult phenotypes of genetic developmental and epileptic encephalopathies.Brain Commun. 2025 Jan 20;7(1):fcaf028. doi: 10.1093/braincomms/fcaf028. eCollection 2025. Brain Commun. 2025. PMID: 39882024 Free PMC article.
-
Developmental and epileptic encephalopathies: recognition and approaches to care.Epileptic Disord. 2021 Feb 1;23(1):40-52. doi: 10.1684/epd.2021.1244. Epileptic Disord. 2021. PMID: 33632673 Review.
-
Case Series of Early SCN1A-Related Developmental and Epileptic Encephalopathies.J Pediatr Neurosci. 2021 Jul-Sep;16(3):212-217. doi: 10.4103/jpn.JPN_99_20. Epub 2021 Jul 2. J Pediatr Neurosci. 2021. PMID: 36160609 Free PMC article.
-
Predictors of genetic diagnosis in individuals with developmental and epileptic encephalopathies.Epilepsy Behav. 2024 Jun;155:109762. doi: 10.1016/j.yebeh.2024.109762. Epub 2024 Apr 18. Epilepsy Behav. 2024. PMID: 38636144
-
[Epileptic encephalopathies of onset in neonates and infants].Medicina (B Aires). 2022 Aug 30;82 Suppl 3:13-18. Medicina (B Aires). 2022. PMID: 36054851 Review. Spanish.
Cited by
-
Developmental and Epileptic Encephalopathy: Pathogenesis of Intellectual Disability Beyond Channelopathies.Biomolecules. 2025 Jan 15;15(1):133. doi: 10.3390/biom15010133. Biomolecules. 2025. PMID: 39858526 Free PMC article. Review.
-
A Case Report of Parental Germline Mosaicism in the PCDH19 Gene of Two Iranian Siblings.Basic Clin Neurosci. 2024 Jul-Aug;15(4):541-552. doi: 10.32598/bcn.2023.5507.1. Epub 2024 Jul 1. Basic Clin Neurosci. 2024. PMID: 39553263 Free PMC article.
-
Neonatal Encephalopathy due to Glutaminase Deficiency in a Neonate.Clin Case Rep. 2024 Nov 17;12(11):e9567. doi: 10.1002/ccr3.9567. eCollection 2024 Nov. Clin Case Rep. 2024. PMID: 39559284 Free PMC article.
References
-
- McTague A. et al. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016;15(3):304–16. - PubMed
-
- Zuberi SM. et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1349–97. - PubMed
-
- Specchio N. et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1398–442. - PubMed
LinkOut - more resources
Full Text Sources
