Research on the mechanism of Guanyu Zhixie Granule in intervening gastric ulcers in rats based on network pharmacology and multi-omics
- PMID: 38835897
- PMCID: PMC11149358
- DOI: 10.3389/fvets.2024.1390473
Research on the mechanism of Guanyu Zhixie Granule in intervening gastric ulcers in rats based on network pharmacology and multi-omics
Abstract
Objective: Guanyu Zhixie Granule (GYZXG) is a traditional Chinese medicine compound with definite efficacy in intervening in gastric ulcers (GUs). However, the effect mechanisms on GU are still unclear. This study aimed to explore its mechanism against GU based on amalgamated strategies.
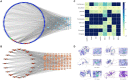
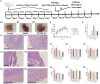
Methods: The comprehensive chemical characterization of the active compounds of GYZXG was conducted using UHPLC-Q/TOF-MS. Based on these results, key targets and action mechanisms were predicted through network pharmacology. GU was then induced in rats using anhydrous ethanol (1 mL/200 g). The intervention effects of GYZXG on GU were evaluated by measuring the inhibition rate of GU, conducting HE staining, and assessing the levels of IL-6, TNF-α, IL-10, IL-4, Pepsin (PP), and epidermal growth factor (EGF). Real-time quantitative PCR (RT-qPCR) was used to verify the mRNA levels of key targets and pathways. Metabolomics, combined with 16S rRNA sequencing, was used to investigate and confirm the action mechanism of GYZXG on GU. The correlation analysis between differential gut microbiota and differential metabolites was conducted using the spearman method.
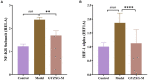
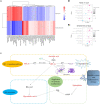
Results: For the first time, the results showed that nine active ingredients and sixteen targets were confirmed to intervene in GU when using GYZXG. Compared with the model group, GYZXG was found to increase the ulcer inhibition rate in the GYZXG-M group (p < 0.05), reduce the levels of IL-6, TNF-α, PP in gastric tissue, and increase the levels of IL-10, IL-4, and EGF. GYZXG could intervene in GU by regulating serum metabolites such as Glycocholic acid, Epinephrine, Ascorbic acid, and Linoleic acid, and by influencing bile secretion, the HIF-1 signaling pathway, and adipocyte catabolism. Additionally, GYZXG could intervene in GU by altering the gut microbiota diversity and modulating the relative abundance of Bacteroidetes, Bacteroides, Verrucomicrobia, Akkermansia, and Ruminococcus. The differential gut microbiota was strongly associated with serum differential metabolites. KEGG enrichment analysis indicated a significant role of the HIF-1 signaling pathway in GYZXG's intervention on GU. The changes in metabolites within metabolic pathways and the alterations in RELA, HIF1A, and EGF mRNA levels in RT-qPCR experiments provide further confirmation of this result.
Conclusion: GYZXG can intervene in GU induced by anhydrous ethanol in rats by regulating gut microbiota and metabolic disorders, providing a theoretical basis for its use in GU intervention.
Keywords: 16S rRNA; Guanyu Zhixie Granules; drug interaction; gastric ulcer; network pharmacology; untargeted metabolomics.
Copyright © 2024 Ma, Ji, Wu, Li, Dong, Yang, Wei and Hua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- Chen WL, Wu ZF, Deng ZY, Hu PY, Wang F, Zheng Q, et al. Status and advance in study on anti-ulcer of traditional Chinese medicine. Chin J Exp Tradit Med Formulae. (2013) 19:362–7. doi: 10.11653/syfj2013080362 - DOI
-
- Hu Y, Xiao JD. A review on treating gastric ulcer in TCM. Clin. J. Chin. Med. (2017) 9:142–5. doi: 10.3969/j.issn.1674-7860.2017.29.071 - DOI
-
- Li J. The Acute Toxicity, Pharmacodynamics Study of Chinese Medicine Compound Guanyuzhixiesan and the Quality Control Study of Its Granulated Dosage Forms Gansu Agricultural University; (2016).
LinkOut - more resources
Full Text Sources
Miscellaneous

