Primary central nervous system lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up
- PMID: 38836097
- PMCID: PMC11148853
- DOI: 10.1002/hem3.89
Primary central nervous system lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up
Erratum in
-
Correction to "Primary central nervous system lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up".Hemasphere. 2024 Jul 22;8(7):e118. doi: 10.1002/hem3.118. eCollection 2024 Jul. Hemasphere. 2024. PMID: 39040837 Free PMC article.
Abstract
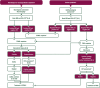
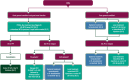
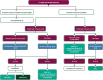
This EHA-ESMO Clinical Practice Guideline provides key recommendations for managing primary DLBCL of the CNS.The guideline covers clinical, imaging and pathological diagnosis, staging and risk assessment, treatment and follow-up.Algorithms for first-line and salvage treatments are provided.The author group encompasses a multidisciplinary group of experts from different institutions and countries in Europe.Recommendations are based on available scientific data and the authors' collective expert opinion.
© 2024 The Authors. HemaSphere Published by John Wiley & Sons on behalf of European Hematology Association and by Elsevier Ltd on behalf of European Society for Medical Oncology.
Conflict of interest statement
AJMF reports personal financial interests for advisory board membership for AbbVie, AstraZeneca, Bristol Myers Squibb (BMS), Genmab, Gilead, Incyte, Juno, Novartis, PletixaPharm and Roche; institutional financial interests as local Principal Investigator (PI) for ADC Therapeutics, Amgen, BeiGene, BMS, Genmab, Gilead, Hutchison Medipharma, Incyte, Janssen, Novartis, Pfizer, Pharmacyclics and Takeda; institution research grants from BTG Therapeutics; institutional funding from Roche; non‐financial interests as a member of the Global Outreach Committee of the EHA and as a member of the Board of Directors (President) of Fondazione Italiana Linfomi. GI reports personal financial interests as an advisory board member for Gilead, Incyte, Roche and as an invited speaker for Riemser; non‐financial interests as a member of Deutsche Gesellschaft für Hämatologie und Onkologie (DGHO) and a leadership role for the German Lymphoma Alliance (Mitglied des Vorstandes). JKD reports personal financial interests as an advisory board member for Eli Lilly. DPA reports non‐financial interests as a member of an academic subcommittee for the British Society of Neuroradiologists. JECB reports personal financial interests as an advisory board member for Gilead and lecture honorarium from Novartis; institutional financial interests for funding of educational symposia from Roche and TEVA. TC reports personal financial interests for advisory board membership and consultancy for Janssen‐Cilag S.p.A; speaking honoraria from Takeda; participation in the Hema for the Future project for Sandoz. KC reports personal financial interests as an advisory board member for AbbVie, Atara, Celgene, Incyte, Janssen, Kite, Roche and Takeda; personal financial interests as an invited speaker for Incyte, Kite, Roche and Takeda; non‐financial interests as a member of the American Society of Clinical Oncology (ASCO) and the EHA, and leadership roles with the National Cancer Research Institute (NCRI; chair of UK NCRI T‐cell lymphoma study group). CPF reports personal financial interests as an advisory board member for AbbVie, AstraZeneca, Atarabio, BMS, Genmab, Gilead/Kite, Incyte, Janssen, Lilly, MorphoSys, Ono, Roche, SERB and Sobi; personal financial interests as an invited speaker for AbbVie, Gilead/Kite, Incyte, Janssen, Roche and Takeda; institutional financial interests as coordinating PI for BeiGene and Roche; institutional financial interests as a steering committee member for Genmab and MorphoSys; non‐financial interests for an advisory role for Blood Cancer UK (clinical trials funding committee member) and Lymphoma Action (medical advisory panel member); non‐financial interests for leadership roles with Cure Leukaemia (clinical trials steering committee member) and the NCRI (chair of UK NCRI aggressive lymphoma study group). KHX reports personal financial interests as an invited speaker for BTG. MP reports personal financial interests as an invited speaker for BeiGene and Novartis; personal financial interests for expert testimony for Ventana Roche. ES reports personal financial interests as an invited speaker for Riemser Pharma; personal financial interests for a writing engagement for Riemser Pharma; personal financial interests as an advisory board member for SERB Pharmaceuticals; institutional financial interests as a coordinating PI for Riemser Pharma and as a local PI for AbbVie, Riemser Pharma and Roche; non‐financial interests as a PI for AbbVie and Roche; non‐financial interests as a member of the DGHO and German Lymphoma Alliance. CS reports institutional funding from AstraZeneca. LS reports personal financial interests as an advisory board member for Kyowa Kirin and Takeda; personal financial interests as author royalties from Munksgaard Publishing and Springer Verlag; institutional financial interests as a steering committee member for Varian and ViewRay; non‐financial interests for leadership roles with the International Lymphoma Radiation Oncology Group (Vice Chair) and the Danish Lymphoma Radiation Oncology Group (Chair); non‐financial interests as a PI for the European Organisation for Research and Treatment of Cancer (EORTC); non‐financial interests as a member of ASCO, American Society for Therapeutic Radiology and Oncology (ASTRO) and European Society for Therapeutic Radiology and Oncology (ESTRO). EZ reports personal financial interests as an advisory board member for AbbVie, BeiGene, BMS, Curis, Eli Lilly, Incyte, Ipsen, Janssen, Merck, Miltenyi Biomedicine and Roche; institutional financial interests for travel grants from BeiGene, Gilead, Janssen and Roche; institutional financial interests for trial sponsorship from AstraZeneca, BeiGene, Celgene/BMS, Incyte, Janssen and Roche. CB reports personal financial interests as an invited speaker for AbbVie, Pfizer and Sobi; personal financial interests as an advisory board member for BeiGene, Celltrion, Gilead Sciences, Incyte, Janssen, MorphoSys, Novartis, Regeneron and Roche; institutional funding from AbbVie, Amgen, Celltrion, Janssen, MSD, Pfizer and Roche. MJ reports personal financial interests as an advisory board member for BMS, Genmab, Gilead, Janssen and Novartis; personal financial interests as an invited speaker for Incyte; institutional financial interests as an invited speaker for Roche; institutional funding from AbbVie, AstraZeneca, Celgene, Gilead, Janssen and Roche; institutional financial interests as coordinating PI for BioInvent. MD reports personal financial interests as an invited speaker for AstraZeneca, Gilead/Kite, Janssen, Novartis and Roche; personal financial interests as an advisory board member for AstraZeneca, BeiGene, BMS/Celgene, Genmab, Gilead, Janssen, Lilly/Loxo, Novartis and Roche; institutional research grants from AbbVie, Bayer, Celgene, Janssen and Roche; institutional funding from Gilead/Kite; non‐financial interests as a member of ASCO, American Society of Hematology (ASH; subcommittee member), DGHO (prior Board member), EHA (Executive Board member), ESMO (Faculty member) and Lymphoma Research Foundation [LRF; Mantle Cell Lymphoma (MCL) Consortium member]. DM has declared no conflicts of interest.
Figures

References
-
- Kluin PM, Deckert M, Ferry JA. Primary diffuse large B‐cell lymphoma of the CNS. In: Swerdlow SH, Campo E, Harris NL, et al. eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2017:300‐302.
-
- Deckert M, Ferry JA. Primary diffuse large B‐cell lymphoma of the CNS. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Central Nervous System Tumours. 5th ed Lyon, France: IARC; 2021:351‐355.
-
- Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187‐194. - PubMed
