Ocular working memory signals are flexible to behavioral priority and subjective imagery strength
- PMID: 38836298
- PMCID: PMC11383386
- DOI: 10.1152/jn.00446.2023
Ocular working memory signals are flexible to behavioral priority and subjective imagery strength
Abstract
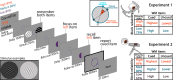
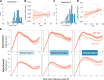
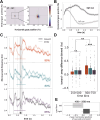
The pupillary light response was long considered a brainstem reflex, outside of cognitive influence. However, newer findings indicate that pupil dilation (and eye movements) can reflect content held "in mind" with working memory (WM). These findings may reshape understanding of ocular and WM mechanisms, but it is unclear whether the signals are artifactual or functional to WM. Here, we ask whether peripheral and oculomotor WM signals are sensitive to the task-relevance or "attentional state" of WM content. During eye-tracking, human participants saw both dark and bright WM stimuli, then were retroactively cued to the item that would most likely be tested. Critically, we manipulated the attentional priority among items by varying the cue reliability across blocks. We confirmed previous findings that remembering darker items is associated with larger pupils (vs. brighter), and that gaze is biased toward cued item locations. Moreover, we discovered that pupil and eye movement responses were influenced differently by WM item relevance. Feature-specific pupillary effects emerged only for highly prioritized WM items but were eliminated when cues were less reliable, and pupil effects also increased with self-reported visual imagery strength. Conversely, gaze position consistently veered toward the cued item location, regardless of cue reliability. However, biased microsaccades occurred at a higher frequency when cues were more reliable, though only during a limited post-cue time window. Therefore, peripheral sensorimotor processing is sensitive to the task-relevance or functional state of internal WM content, but pupillary and eye movement WM signals show distinct profiles. These results highlight a potential role for early visual processing in maintaining multiple WM content dimensions.NEW & NOTEWORTHY Here, we found that working memory (WM)-driven ocular inflections-feature-specific pupillary and saccadic biases-were muted for memory items that were less behaviorally relevant. This work illustrates that functionally informative goal signals may extend as early as the sensorimotor periphery, that pupil size may be under more fine-grained control than originally thought, and that ocular signals carry multiple dimensions of cognitively relevant information.
Keywords: eye movements; pupillometry; visual attention; visual imagery; visual working memory.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Naturalistic Eye Movement Tasks in Parkinson's Disease: A Systematic Review.J Parkinsons Dis. 2024;14(7):1369-1386. doi: 10.3233/JPD-240092. J Parkinsons Dis. 2024. PMID: 39422967 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Susceptibility to visual interference in working memory: Different results depending on the prioritization mode?J Exp Psychol Hum Percept Perform. 2025 Jun;51(6):791-807. doi: 10.1037/xhp0001315. Epub 2025 Apr 7. J Exp Psychol Hum Percept Perform. 2025. PMID: 40193522
Cited by
-
Attentional shifts bias microsaccade direction but do not cause new microsaccades.Commun Psychol. 2024 Oct 22;2(1):97. doi: 10.1038/s44271-024-00149-7. Commun Psychol. 2024. PMID: 39438653 Free PMC article.
References
-
- Einhäuser W. The pupil as marker of cognitive processes. In: Computational and Cognitive Neuroscience of Vision, edited by Zhao Q. Singapore: Springer, 2017, p. 141–169. doi: 10.1007/978-981-10-0213-7_7. - DOI
-
- Vernet M, Quentin R, Chanes L, Mitsumasu A, Valero-Cabré A. Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci 8: 66, 2014. doi: 10.3389/fnint.2014.00066. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

