Vascular Damage and Repair - Are Small-Diameter Vascular Grafts Still the "Holy Grail" of Tissue Engineering?
- PMID: 38836460
- PMCID: PMC11412351
- DOI: 10.33549/physiolres.935294
Vascular Damage and Repair - Are Small-Diameter Vascular Grafts Still the "Holy Grail" of Tissue Engineering?
Abstract
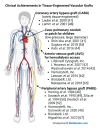
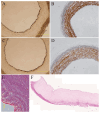
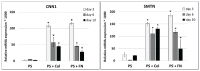
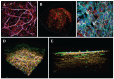
Cardiovascular diseases are the most important cause of morbidity and mortality in the civilized world. Stenosis or occlusion of blood vessels leads not only to events that are directly life-threatening, such as myocardial infarction or stroke, but also to a significant reduction in quality of life, for example in lower limb ischemia as a consequence of metabolic diseases. The first synthetic polymeric vascular replacements were used clinically in the early 1950s. However, they proved to be suitable only for larger-diameter vessels, where the blood flow prevents the attachment of platelets, pro-inflammatory cells and smooth muscle cells on their inner surface, whereas in smaller-diameter grafts (6 mm or less), these phenomena lead to stenosis and failure of the graft. Moreover, these polymeric vascular replacements, like biological grafts (decellularized or devitalized), are cell-free, i.e. there are no reconstructed physiological layers of the blood vessel wall, i.e. an inner layer of endothelial cells to prevent thrombosis, a middle layer of smooth muscle cells to perform the contractile function, and an outer layer to provide innervation and vascularization of the vessel wall. Vascular substitutes with these cellular components can be constructed by tissue engineering methods. However, it has to be admitted that even about 70 years after the first polymeric vascular prostheses were implanted into human patients, there are still no functional small-diameter vascular grafts on the market. The damage to small-diameter blood vessels has to be addressed by endovascular approaches or by autologous vascular substitutes, which leads to some skepticism about the potential of tissue engineering. However, new possibilities of this approach lie in the use of modern technologies such as 3D bioprinting and/or electrospinning in combination with stem cells and pre-vascularization of tissue-engineered vascular grafts. In this endeavor, sex-related differences in the removal of degradable biomaterials by the cells and in the behavior of stem cells and pre-differentiated vascular cells need to be taken into account. Key words: Blood vessel prosthesis, Regenerative medicine, Stem cells, Footprint-free iPSCs, sr-RNA, Dynamic bioreactor, Sex-related differences.
Conflict of interest statement
Figures

Similar articles
-
Biofabrication of small diameter tissue-engineered vascular grafts.Acta Biomater. 2022 Jan 15;138:92-111. doi: 10.1016/j.actbio.2021.11.012. Epub 2021 Nov 13. Acta Biomater. 2022. PMID: 34781026 Review.
-
Perfusion Bioreactor Conditioning of Small-diameter Plant-based Vascular Grafts.Tissue Eng Regen Med. 2024 Dec;21(8):1189-1201. doi: 10.1007/s13770-024-00670-0. Epub 2024 Oct 1. Tissue Eng Regen Med. 2024. PMID: 39354262 Free PMC article.
-
Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review.Cardiovasc Eng Technol. 2020 Oct;11(5):495-521. doi: 10.1007/s13239-020-00482-y. Epub 2020 Aug 18. Cardiovasc Eng Technol. 2020. PMID: 32812139 Review.
-
Tissue engineered small-diameter vascular grafts.Clin Plast Surg. 2003 Oct;30(4):507-17. doi: 10.1016/s0094-1298(03)00069-5. Clin Plast Surg. 2003. PMID: 14621299 Review.
-
The surrounding tissue contributes to smooth muscle cells' regeneration and vascularization of small diameter vascular grafts.Biomater Sci. 2019 Feb 26;7(3):914-925. doi: 10.1039/c8bm01277f. Biomater Sci. 2019. PMID: 30511718
References
-
- Gupta S, Khanal S, Bhavnani N, Mathias A, Lallo J, Kiriakou A, Ferrell J, Raman P. Sex-specific differences in atherosclerosis, thrombospondin-1, and smooth muscle cell differentiation in metabolic syndrome versus non-metabolic syndrome mice. Front Cardiovasc Med. 2022;9:1020006. doi: 10.3389/fcvm.2022.1020006. - DOI - PMC - PubMed
-
- World Health Organization. Cardiovascular diseases (CVDs) Jun 11, 2021. [accessed December 10, 2023]. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...
-
- OECD. European Observatory on Health Systems and Policies. Czechia: Country Health Profile 2021 OECD; 2021. [accessed December 10, 2023]. https://health.ec.europa.eu/system/files/2021-12/2021_chp_cs_english.pdf .
-
- Bacakova L, Travnickova M, Filova E, Matějka R, Stepanovska J, Musilkova J, Zarubova J, Molitor M. The role of vascular smooth muscle cells in the physiology and pathophysiology of blood vessels. In: Sakuma K, editor. Muscle Cell and Tissue - Current Status of Research Field. IntechOpen; London, United Kingdom: 2018. pp. 229–256. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
