From the Pineal Gland to the Central Clock in the Brain: Beginning of Studies of the Mammalian Biological Rhythms in the Institute of Physiology of the Czech Academy of Sciences
- PMID: 38836462
- PMCID: PMC11412352
- DOI: 10.33549/physiolres.935377
From the Pineal Gland to the Central Clock in the Brain: Beginning of Studies of the Mammalian Biological Rhythms in the Institute of Physiology of the Czech Academy of Sciences
Abstract
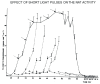
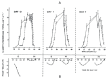
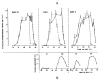
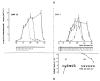
The Institute of Physiology of the Czech Academy of Sciences (CAS) has been involved in the field of chronobiology, i.e., in research on temporal regulation of physiological processes, since 1970. The review describes the first 35 years of the research mostly on the effect of light and daylength, i.e., photoperiod, on entrainment or resetting of the pineal rhythm in melatonin production and of intrinsic rhythms in the central biological clock. This clock controls pineal and other circadian rhythms and is located in the suprachiasmatic nuclei (SCN) of the hypothalamus. During the early chronobiological research, many original findings have been reported, e.g. on mechanisms of resetting of the pineal rhythm in melatonin production by short light pulses or by long exposures of animals to light at night, on modulation of the nocturnal melatonin production by the photoperiod or on the presence of high affinity melatonin binding sites in the SCN. The first evidence was given that the photoperiod modulates functional properties of the SCN and hence the SCN not only controls the daily programme of the organism but it may serve also as a calendar measuring the time of a year. During all the years, the chronobiological community has started to talk about "the Czech school of chronobiology". At present, the today´s Laboratory of Biological Rhythms of the Institute of Physiology CAS continues in the chronobiological research and the studies have been extended to the entire circadian timekeeping system in mammals with focus on its ontogenesis, entrainment mechanisms and circadian regulation of physiological functions. Key words: Pineal, Melatonin, AA-NAT rhythm, Light entrainment, Photoperiod, SCN clock.
Conflict of interest statement
Figures









Similar articles
-
The photoperiod, circadian regulation and chronodisruption: the requisite interplay between the suprachiasmatic nuclei and the pineal and gut melatonin.J Physiol Pharmacol. 2011 Jun;62(3):269-74. J Physiol Pharmacol. 2011. PMID: 21893686 Review.
-
Circadian actions of melatonin at the suprachiasmatic nucleus.Behav Brain Res. 1996;73(1-2):135-9. doi: 10.1016/0166-4328(96)00085-x. Behav Brain Res. 1996. PMID: 8788491 Review.
-
Transcriptomic Plasticity of the Circadian Clock in Response to Photoperiod: A Study in Male Melatonin-Competent Mice.J Biol Rhythms. 2024 Oct;39(5):423-439. doi: 10.1177/07487304241265439. Epub 2024 Aug 2. J Biol Rhythms. 2024. PMID: 39096022 Free PMC article.
-
The melatonin rhythm: both a clock and a calendar.Experientia. 1993 Aug 15;49(8):654-64. doi: 10.1007/BF01923947. Experientia. 1993. PMID: 8395408 Review.
-
A mathematical model of melatonin synthesis and interactions with the circadian clock.Math Biosci. 2024 Nov;377:109280. doi: 10.1016/j.mbs.2024.109280. Epub 2024 Sep 6. Math Biosci. 2024. PMID: 39243938
References
-
- Fiske VM, Bryant GK, Putnam J. Effect of light on the weight of the pineal in the rat. Endocrinology. 1960;66:489–491. doi: 10.1210/endo-66-3-489. - DOI
-
- Quay WB. Cellular and Physiological Mechanisms. Charles C Thomas; Springfield, Illinois: 1974. Pineal Chemistry; pp. 1–430.
-
- Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587–2591. doi: 10.1021/ja01543a060. - DOI
-
- Lerner AB, Case JD, Heinzelman RV. Structure of melatonin. J Am Chem Soc. 1959;81:6084–6088. doi: 10.1021/ja01531a060. - DOI
-
- Arendt J. Melatonin and the Mammalian Pineal Gland. Chapman C Hall; London: 1995. pp. 1–321.
