What Is the Potential Value of a Randomized Trial of Different Thresholds to Initiate Invasive Ventilation? A Health Economic Analysis
- PMID: 38836575
- PMCID: PMC11152783
- DOI: 10.1097/CCE.0000000000001098
What Is the Potential Value of a Randomized Trial of Different Thresholds to Initiate Invasive Ventilation? A Health Economic Analysis
Abstract
Objectives: To estimate the expected value of undertaking a future randomized controlled trial of thresholds used to initiate invasive ventilation compared with usual care in hypoxemic respiratory failure.
Perspective: Publicly funded healthcare payer.
Setting: Critical care units capable of providing invasive ventilation and unconstrained by resource limitations during usual (nonpandemic) practice.
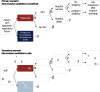
Methods: We performed a model-based cost-utility estimation with individual-level simulation and value-of-information analysis focused on adults, admitted to critical care, receiving noninvasive oxygen. In the primary scenario, we compared hypothetical threshold A to usual care, where threshold A resulted in increased use of invasive ventilation and improved survival compared with usual care. In the secondary scenario, we compared hypothetical threshold B to usual care, where threshold B resulted in decreased use of invasive ventilation and similar survival compared with usual care. We assumed a willingness-to-pay of 100,000 Canadian dollars (CADs) per quality-adjusted life year.
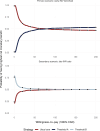
Results: In the primary scenario, threshold A was cost-effective compared with usual care due to improved hospital survival (78.1% vs. 75.1%), despite more use of invasive ventilation (62% vs. 30%) and higher lifetime costs (86,900 vs. 75,500 CAD). In the secondary scenario, threshold B was cost-effective compared with usual care due to similar survival (74.5% vs. 74.6%) with less use of invasive ventilation (20.2% vs. 27.6%) and lower lifetime costs (71,700 vs. 74,700 CAD). Value-of-information analysis showed that the expected value to Canadian society over 10 years of a 400-person randomized trial comparing a threshold for invasive ventilation to usual care in hypoxemic respiratory failure was 1.35 billion CAD or more in both scenarios.
Conclusions: It would be highly valuable to society to identify thresholds that, in comparison to usual care, either increase survival or reduce invasive ventilation without reducing survival.
Copyright © 2024 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Dr. Yarnell was funded by the Canadian Institutes for Health Research Vanier Scholar program, the Eliot Phillipson Clinician Scientist Training Program, and the Clinician Investigator Program of the University of Toronto. Dr. Heath is supported by a Canada Research Chair in Statistical Trial Design and funded by the Discovery Grant Program of the Natural Sciences and Engineering Research Council of Canada (RGPIN-2021-03366). Dr. Sung is supported by the Canada Research Chair in Pediatric Oncology Supportive Care. Dr. Fowler is the H. Barrie Fairley Professor of Critical Care at the University Health Network, Interdepartmental Division of Critical Care Medicine, and University of Toronto. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures


Similar articles
-
Proportional-assist ventilation with load-adjustable gain factors for mechanical ventilation: a cost-utility analysis.CMAJ Open. 2022 Feb 15;10(1):E126-E135. doi: 10.9778/cmajo.20210078. Print 2022 Jan-Mar. CMAJ Open. 2022. PMID: 35168935 Free PMC article.
-
Cost-effectiveness of Out-of-Hospital Continuous Positive Airway Pressure for Acute Respiratory Failure.Ann Emerg Med. 2015 May;65(5):556-563.e6. doi: 10.1016/j.annemergmed.2014.12.028. Epub 2015 Feb 27. Ann Emerg Med. 2015. PMID: 25737210 Free PMC article.
-
Cost-effectiveness of exogenous surfactant therapy in pediatric patients with acute hypoxemic respiratory failure.Pediatr Crit Care Med. 2005 Mar;6(2):160-5. doi: 10.1097/01.PCC.0000154965.08432.16. Pediatr Crit Care Med. 2005. PMID: 15730602
-
Reverse Total Shoulder Arthroplasty Is the Most Cost-effective Treatment Strategy for Proximal Humerus Fractures in Older Adults: A Cost-utility Analysis.Clin Orthop Relat Res. 2022 Oct 1;480(10):2013-2026. doi: 10.1097/CORR.0000000000002219. Epub 2022 May 4. Clin Orthop Relat Res. 2022. PMID: 35507306 Free PMC article.
-
Economics of mechanical ventilation and respiratory failure.Crit Care Clin. 2012 Jan;28(1):39-55, vi. doi: 10.1016/j.ccc.2011.10.004. Crit Care Clin. 2012. PMID: 22123098 Review.
References
-
- Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 - PubMed
-
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force: Acute respiratory distress syndrome. JAMA 2012; 307:2526–2533 - PubMed
-
- Herridge MS, Tansey CM, Matté A, et al. ; Canadian Critical Care Trials Group: Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364:1293–1304 - PubMed
-
- Cook T, Woodall N, Frerk C: The NAP4 Report: Major Complications of Airway Management in the UK. London, United Kingdom, Royal College of Anaesthetists, 2011. Available at: https://www.rcoa.ac.uk/research/research-projects/national-audit-project.... Accessed May 22, 2024 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

