The HERCulean task of recognizing, ubiquitinating, and shielding misfolded integral membrane proteins
- PMID: 38836811
- PMCID: PMC11153772
- DOI: 10.1083/jcb.202405160
The HERCulean task of recognizing, ubiquitinating, and shielding misfolded integral membrane proteins
Abstract
During ER-associated decay, unfolded membrane-resident proteins are targeted for removal and degradation by ubiquitin ligases whose identities and precise operations remain unclear. In this issue, Guerriero and Brodsky discuss new results from Kamada et al. (https://doi.org/10.1083/jcb.202308003) showing the clearance of misfolded CFTR by the E3 ligase HERC3.
© 2024 Guerriero and Brodsky.
Figures
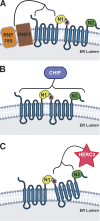
Comment on
-
HERC3 facilitates ERAD of select membrane proteins by recognizing membrane-spanning domains.J Cell Biol. 2024 Jul 1;223(7):e202308003. doi: 10.1083/jcb.202308003. Epub 2024 May 9. J Cell Biol. 2024. PMID: 38722278 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

