A parameterized two-domain thermodynamic model explains diverse mutational effects on protein allostery
- PMID: 38836839
- PMCID: PMC11152574
- DOI: 10.7554/eLife.92262
A parameterized two-domain thermodynamic model explains diverse mutational effects on protein allostery
Abstract
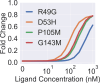
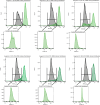
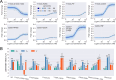
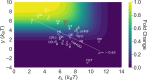
New experimental findings continue to challenge our understanding of protein allostery. Recent deep mutational scanning study showed that allosteric hotspots in the tetracycline repressor (TetR) and its homologous transcriptional factors are broadly distributed rather than spanning well-defined structural pathways as often assumed. Moreover, hotspot mutation-induced allostery loss was rescued by distributed additional mutations in a degenerate fashion. Here, we develop a two-domain thermodynamic model for TetR, which readily rationalizes these intriguing observations. The model accurately captures the in vivo activities of various mutants with changes in physically transparent parameters, allowing the data-based quantification of mutational effects using statistical inference. Our analysis reveals the intrinsic connection of intra- and inter-domain properties for allosteric regulation and illustrate epistatic interactions that are consistent with structural features of the protein. The insights gained from this study into the nature of two-domain allostery are expected to have broader implications for other multi-domain allosteric proteins.
Keywords: Bayesian inference; E. coli; allosteric hotspots; allostery; epistasis; molecular biophysics; structural biology; thermodynamic model.
© 2023, Liu et al.
Conflict of interest statement
ZL, TG, SR No competing interests declared, QC Senior editor, eLife
Figures
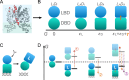



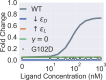
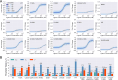

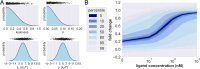
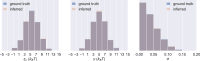
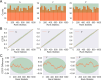
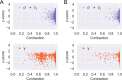
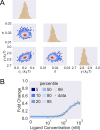


Update of
-
A parametrized two-domain thermodynamic model explains diverse mutational effects on protein allostery.bioRxiv [Preprint]. 2024 Feb 14:2023.08.06.552196. doi: 10.1101/2023.08.06.552196. bioRxiv. 2024. Update in: Elife. 2024 Jun 05;12:RP92262. doi: 10.7554/eLife.92262. PMID: 37662419 Free PMC article. Updated. Preprint.
Similar articles
-
A parametrized two-domain thermodynamic model explains diverse mutational effects on protein allostery.bioRxiv [Preprint]. 2024 Feb 14:2023.08.06.552196. doi: 10.1101/2023.08.06.552196. bioRxiv. 2024. Update in: Elife. 2024 Jun 05;12:RP92262. doi: 10.7554/eLife.92262. PMID: 37662419 Free PMC article. Updated. Preprint.
-
Molecular Dynamics Simulations Establish the Molecular Basis for the Broad Allostery Hotspot Distributions in the Tetracycline Repressor.J Am Chem Soc. 2022 Jun 22;144(24):10870-10887. doi: 10.1021/jacs.2c03275. Epub 2022 Jun 8. J Am Chem Soc. 2022. PMID: 35675441 Free PMC article.
-
The induction of folding cooperativity by ligand binding drives the allosteric response of tetracycline repressor.Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22263-8. doi: 10.1073/pnas.0911566106. Epub 2009 Dec 22. Proc Natl Acad Sci U S A. 2009. PMID: 20080791 Free PMC article.
-
Ensemble allosteric model: energetic frustration within the intrinsically disordered glucocorticoid receptor.Philos Trans R Soc Lond B Biol Sci. 2018 Jun 19;373(1749):20170175. doi: 10.1098/rstb.2017.0175. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29735729 Free PMC article. Review.
-
Regulating transcription regulators via allostery and flexibility.Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22035-6. doi: 10.1073/pnas.0912300107. Epub 2009 Dec 23. Proc Natl Acad Sci U S A. 2009. PMID: 20080782 Free PMC article. Review. No abstract available.
Cited by
-
Identification and understanding of allostery hotspots in proteins: Integration of deep mutational scanning and multi-faceted computational analyses.J Mol Biol. 2025 Feb 12:168998. doi: 10.1016/j.jmb.2025.168998. Online ahead of print. J Mol Biol. 2025. PMID: 39952349 Review.
-
Modulation of Allostery with Multiple Mechanisms by Hotspot Mutations in TetR.bioRxiv [Preprint]. 2023 Dec 12:2023.08.29.555381. doi: 10.1101/2023.08.29.555381. bioRxiv. 2023. Update in: J Am Chem Soc. 2024 Jan 31;146(4):2757-2768. doi: 10.1021/jacs.3c12494. PMID: 37905112 Free PMC article. Updated. Preprint.
-
The value of protein allostery in rational anticancer drug design: an update.Expert Opin Drug Discov. 2024 Sep;19(9):1071-1085. doi: 10.1080/17460441.2024.2384467. Epub 2024 Jul 28. Expert Opin Drug Discov. 2024. PMID: 39068599 Review.
-
Machine learning in molecular biophysics: Protein allostery, multi-level free energy simulations, and lipid phase transitions.Biophys Rev (Melville). 2025 Feb 12;6(1):011305. doi: 10.1063/5.0248589. eCollection 2025 Mar. Biophys Rev (Melville). 2025. PMID: 39957913 Review.
-
Entropy tree networks of residue dynamics encode protein allostery.bioRxiv [Preprint]. 2025 Jul 1:2025.05.28.656549. doi: 10.1101/2025.05.28.656549. bioRxiv. 2025. PMID: 40501711 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

