Two doses of fosaprepitant included prophylactic treatment for the three-day cisplatin-based chemotherapy induced nausea and vomiting
- PMID: 38836908
- PMCID: PMC11153275
- DOI: 10.1007/s00432-024-05766-7
Two doses of fosaprepitant included prophylactic treatment for the three-day cisplatin-based chemotherapy induced nausea and vomiting
Abstract
Purpose: Neurokinin 1 receptor antagonists included prophylactic treatment was recommended for patients who receive one-day cisplatin chemotherapy. It is unclear whether the prolonged administration of fosaprepitant is effective for three-day cisplatin-based chemotherapy induced nausea and vomiting (CINV). We aim to explore the prophylactic antiemetic efficacy and safety of two doses of fosaprepitant included regimen in the patients receiving multiple-day cisplatin chemotherapy.
Methods: This randomized, parallel-group, open-labelled study was conducted in nine hospitals between February 2021 and February 2023. Patients diagnosed as lung cancer and chemotherapy naive were screened. Eligible participants were scheduled to be treated with highly emetogenic chemotherapy regimen which including three days of cisplatin. Then they were randomly divided into the experimental group (two doses of fosaprepitant, Group 2DF) and the control group (one dose of fosaprepitant, Group C). The primary endpoints included the safety and the average none CINV days (NCDs). This study was registered on the website of chictr.org.cn, number ChiCTR2100042665.
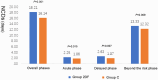
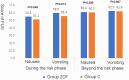
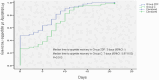
Results: Overall, 204 participants were randomly assigned, and 198 patients were analyzed. No statistical difference in adverse events was found between the two groups. All treatment-related adverse effects for fosaprepitant observed were of grade 1-2. The average NCDs of Group 2DF was significantly more than Group C (18.21 ± 3.40 days vs 16.14 ± 5.20 days, P = 0.001). Furthermore, the better life function score was achieved in Group 2DF according to FLIE questionnaire.
Conclusion: The administration of two-dose fosaprepitant was safe and more effective than one dose in protecting patients from CINV induced by three-day cisplatin included chemotherapy.
Keywords: CINV; Fosaprepitant; Three-day cisplatin; Two doses.
© 2024. The Author(s).
Conflict of interest statement
There is no conflict of interest.
Figures
Similar articles
-
Efficacy, safety and feasibility of fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in pediatric patients receiving moderately and highly emetogenic chemotherapy - results of a non-interventional observation study.BMC Cancer. 2019 Nov 15;19(1):1118. doi: 10.1186/s12885-019-6252-6. BMC Cancer. 2019. PMID: 31730451 Free PMC article.
-
Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: results of a randomized, double-blind phase III trial.Ann Oncol. 2016 Jan;27(1):172-8. doi: 10.1093/annonc/mdv482. Epub 2015 Oct 8. Ann Oncol. 2016. PMID: 26449391 Free PMC article. Clinical Trial.
-
Efficacy, Safety And Feasibility Of Antiemetic Prophylaxis With Fosaprepitant, Granisetron And Dexamethasone In Pediatric Patients With Hemato-Oncological Malignancies.Drug Des Devel Ther. 2019 Sep 30;13:3439-3451. doi: 10.2147/DDDT.S214264. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31686784 Free PMC article.
-
The clinical research study for fosaprepitant to prevent chemotherapy-induced nausea and vomiting: A review.Adv Clin Exp Med. 2023 Jun;32(6):701-706. doi: 10.17219/acem/157061. Adv Clin Exp Med. 2023. PMID: 37026971 Review.
-
Aprepitant and fosaprepitant: a 10-year review of efficacy and safety.Oncologist. 2015 Apr;20(4):450-8. doi: 10.1634/theoncologist.2014-0229. Epub 2015 Mar 20. Oncologist. 2015. PMID: 25795636 Free PMC article. Review.
Cited by
-
Nausea and vomiting in an evolving anticancer treatment landscape: long-delayed and emetogenic antibody-drug conjugates.Future Oncol. 2025 Apr;21(10):1261-1272. doi: 10.1080/14796694.2025.2479417. Epub 2025 Mar 19. Future Oncol. 2025. PMID: 40105595 Free PMC article. Review.
-
Neurokinin-1 receptor antagonists in the current management of chemotherapy-induced nausea and vomiting.Front Med. 2025 Aug;19(4):600-611. doi: 10.1007/s11684-025-1140-8. Epub 2025 Jul 5. Front Med. 2025. PMID: 40616753 Review.
References
-
- Abdel-Malek R, Abbas N, Shohdy KS et al (2017) Addition of 3-day aprepitant to ondansetron and dexamethasone for prophylaxis of chemotherapy-induced nausea and vomiting among patients with diffuse large B cell lymphoma receiving 5-day cisplatin-based chemotherapy. J Egypt Natl Canc Inst 29:155–158 - PubMed
-
- Albany C, Brames MJ, Fausel C, Johnson CS, Picus J, Einhorn LH (2012) Randomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a hoosier oncology group study. J Clin Oncol 30:3998–4003 - PubMed
-
- Funai K, Takamochi K, Itaya T et al (2010) Feasibility study of adjuvant chemotherapy with gemcitabine and split-dose cisplatin for completely resected non-small-cell lung cancer. Lung Cancer 68:78–83 - PubMed
-
- Furugaki K, Yoshida J, Ogawa R et al (2009) Chemotherapy for non-small cell lung cancer: split-dose administration of Cisplatin over four consecutive days and Vinorelbine ditartrate. Gan to Kagaku Ryoho Cancer & Chemother 36:259–263 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical