A Framework for the Administration of Anti-amyloid Monoclonal Antibody Treatments in Early-Stage Alzheimer's Disease
- PMID: 38836991
- PMCID: PMC11182839
- DOI: 10.1007/s40263-024-01097-w
A Framework for the Administration of Anti-amyloid Monoclonal Antibody Treatments in Early-Stage Alzheimer's Disease
Abstract
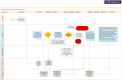
The US Food and Drug Administration (FDA) approval of lecanemab for early-stage Alzheimer's disease (AD) represents an exciting new chapter in the management of neurodegenerative disease, but likewise presents numerous clinical, technical, and financial logistical challenges for both academic and non-academic medical institutions hoping to administer this drug. Minimal resources exist that provide guidance for establishing and maintaining a lecanemab treatment program at the institutional level. The current report aims to provide healthcare institutions a framework for the planning, onboarding, and longitudinal treatment of AD with anti-amyloid monoclonal antibody treatments. We present an implementation study involving three stages: (1) feasibility assessment, (2) operations and going live, and (3) monitoring assessment. We found that implementation of lecanemab in clinical practice was feasible due to the assignment of an enterprise-wide project manager to facilitate the planning phase, a cost analysis showing that lecanemab was financially sustainable, and the development of electronic medical record tools to support operational efficiency.
© 2024. The Author(s).
Conflict of interest statement
Dr. Rosenbloom has served as a consultant for Eisai. Tricia O’Doohue has no conflicts to declare. Lauren Shakalis has no conflicts to declare. Deepashni Mala has no conflicts to declare. Andrew Frazier has no conflicts to declare. Michael Tarrant has no conflicts to declare. Michelle Modrijan has no conflicts to declare. Melora Riveira has no conflicts to declare. Darla Chapman has no conflicts to declare. Yvonne Griffin has no conflicts to declare. Domi Zhou-Clark has no conflicts to declare. Thomas J. Grabowski has no conflicts to declare.
Figures
References
-
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598–695. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical