Drug delivery to and through the skin
- PMID: 38837116
- PMCID: PMC11208237
- DOI: 10.1007/s13346-024-01614-w
Drug delivery to and through the skin
Abstract
Drug delivery technology has advanced significantly over >50 years, and has produced remarkable innovation, countless publications and conferences, and generations of talented and creative scientists. However, a critical review of the current state-of-the-art reveals that the translation of clever and sophisticated drug delivery technologies into products, which satisfy important, unmet medical needs and have been approved by the regulatory agencies, has - given the investment made in terms of time and money - been relatively limited. Here, this point of view is illustrated using a case study of technology for drug delivery into and through the skin and aims: to examine the historical development of this field and the current state-of-the-art; to understand why the translation of drug delivery technologies into products that improve clinical outcomes has been quite slow and inefficient; and to suggest how the impact of technology may be increased and the process of concept to approved product accelerated.
Keywords: Drug delivery technology; Skin barrier function; Skin penetration enhancement – chemical; Skin penetration enhancement – physical; Topical and transdermal drug delivery; Topical formulations and metamorphosis.
© 2024. The Author(s).
Conflict of interest statement
None.
Figures
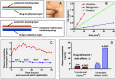
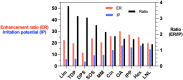

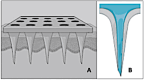


References
-
- Guy RH, Davis AF. Topical drug delivery. In: Griffiths CEM, Barker J, Bleiker TO, Hussain W, Simpson RC, editors. In Rook’s Textbook of Dermatology. Chichester, U.K.: John Wiley & Sons Ltd,; 2024.
-
- Elias PM. Epidermal barrier function: intercellular lamellar lipid structures, origin, composition and metabolism. J Control Release. 1991;15:199–208.
-
- Madison KC. Barrier function of the skin: "la raison d'être" of the epidermis. J Invest Dermatol. 2003;121:231–241. - PubMed
-
- Kasting GB, Smith RL, Cooper ER. Effect of lipid solubility and molecular size on percutaneous absorption. In: Shroot B, Schaefer H, editors. Skin Pharmacokinetics. Basel: Karger; 1987. pp. 138–153.
-
- Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

