Genetic Testing for Global Developmental Delay in Early Childhood
- PMID: 38837156
- PMCID: PMC11154162
- DOI: 10.1001/jamanetworkopen.2024.15084
Genetic Testing for Global Developmental Delay in Early Childhood
Abstract
Importance: Global developmental delay (GDD) is characterized by a complex etiology, diverse phenotypes, and high individual heterogeneity, presenting challenges for early clinical etiologic diagnosis. Cognitive impairment is the core symptom, and despite the pivotal role of genetic factors in GDD development, the understanding of them remains limited.
Objectives: To assess the utility of genetic detection in patients with GDD and to examine the potential molecular pathogenesis of GDD to identify targets for early intervention.
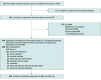
Design, setting, and participants: This multicenter, prospective cohort study enrolled patients aged 12 to 60 months with GDD from 6 centers in China from July 4, 2020, to August 31, 2023. Participants underwent trio whole exome sequencing (trio-WES) coupled with copy number variation sequencing (CNV-seq). Bioinformatics analysis was used to unravel pathogenesis and identify therapeutic targets.
Main outcomes and measures: The main outcomes of this study involved enhancing the rate of positive genetic diagnosis for GDD, broadening the scope of genetic testing indications, and investigating the underlying pathogenesis. The classification of children into levels of cognitive impairment was based on the developmental quotient assessed using the Gesell scale.
Results: The study encompassed 434 patients with GDD (262 [60%] male; mean [SD] age, 25.75 [13.24] months) with diverse degrees of cognitive impairment: mild (98 [23%]), moderate (141 [32%]), severe (122 [28%]), and profound (73 [17%]). The combined use of trio-WES and CNV-seq resulted in a 61% positive detection rate. Craniofacial abnormalities (odds ratio [OR], 2.27; 95% CI, 1.45-3.56), moderate or severe cognitive impairment (OR, 1.69; 95% CI, 1.05-2.70), and age between 12 and 24 months (OR, 1.57; 95% CI, 1.05-2.35) were associated with a higher risk of carrying genetic variants. Additionally, bioinformatics analysis suggested that genetic variants may induce alterations in brain development and function, which may give rise to cognitive impairment. Moreover, an association was found between the dopaminergic pathway and cognitive impairment.
Conclusions and relevance: In this cohort study of patients with GDD, combining trio-WES with CNV-seq was a demonstrable, instrumental strategy for advancing the diagnosis of GDD. The close association among genetic variations, brain development, and clinical phenotypes contributed valuable insights into the pathogenesis of GDD. Notably, the dopaminergic pathway emerged as a promising focal point for potential targets in future precision medical interventions for GDD.
Conflict of interest statement
Figures


References
-
- GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137-150. doi:10.1016/S2215-0366(21)00395-3 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

