Delays to Antibiotics in the Emergency Department and Risk of Mortality in Children With Sepsis
- PMID: 38837160
- PMCID: PMC11154154
- DOI: 10.1001/jamanetworkopen.2024.13955
Delays to Antibiotics in the Emergency Department and Risk of Mortality in Children With Sepsis
Erratum in
-
Error in Results.JAMA Netw Open. 2024 Dec 2;7(12):e2455269. doi: 10.1001/jamanetworkopen.2024.55269. JAMA Netw Open. 2024. PMID: 39680413 Free PMC article. No abstract available.
Abstract
Importance: Pediatric consensus guidelines recommend antibiotic administration within 1 hour for septic shock and within 3 hours for sepsis without shock. Limited studies exist identifying a specific time past which delays in antibiotic administration are associated with worse outcomes.
Objective: To determine a time point for antibiotic administration that is associated with increased risk of mortality among pediatric patients with sepsis.
Design, setting, and participants: This retrospective cohort study used data from 51 US children's hospitals in the Improving Pediatric Sepsis Outcomes collaborative. Participants included patients aged 29 days to less than 18 years with sepsis recognized within 1 hour of emergency department arrival, from January 1, 2017, through December 31, 2021. Piecewise regression was used to identify the inflection point for sepsis-attributable 3-day mortality, and logistic regression was used to evaluate odds of sepsis-attributable mortality after adjustment for potential confounders. Data analysis was performed from March 2022 to February 2024.
Exposure: The number of minutes from emergency department arrival to antibiotic administration.
Main outcomes and measures: The primary outcome was sepsis-attributable 3-day mortality. Sepsis-attributable 30-day mortality was a secondary outcome.
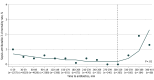
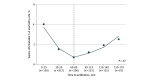
Results: A total of 19 515 cases (median [IQR] age, 6 [2-12] years) were included. The median (IQR) time to antibiotic administration was 69 (47-116) minutes. The estimated time to antibiotic administration at which 3-day sepsis-attributable mortality increased was 330 minutes. Patients who received an antibiotic in less than 330 minutes (19 164 patients) had sepsis-attributable 3-day mortality of 0.5% (93 patients) and 30-day mortality of 0.9% (163 patients). Patients who received antibiotics at 330 minutes or later (351 patients) had 3-day sepsis-attributable mortality of 1.2% (4 patients), 30-day mortality of 2.0% (7 patients), and increased adjusted odds of mortality at both 3 days (odds ratio, 3.44; 95% CI, 1.20-9.93; P = .02) and 30 days (odds ratio, 3.63; 95% CI, 1.59-8.30; P = .002) compared with those who received antibiotics within 330 minutes.
Conclusions and relevance: In this cohort of pediatric patients with sepsis, 3-day and 30-day sepsis-attributable mortality increased with delays in antibiotic administration 330 minutes or longer from emergency department arrival. These findings are consistent with the literature demonstrating increased pediatric sepsis mortality associated with antibiotic administration delay. To guide the balance of appropriate resource allocation with time for adequate diagnostic evaluation, further research is needed into whether there are subpopulations, such as those with shock or bacteremia, that may benefit from earlier antibiotics.
Conflict of interest statement
Figures
Comment in
-
The Quest for Evidence on Time to Antibiotics in Children With Sepsis-Finding the Sweet Spot.JAMA Netw Open. 2024 Jun 3;7(6):e2413926. doi: 10.1001/jamanetworkopen.2024.13926. JAMA Netw Open. 2024. PMID: 38837164 No abstract available.
References
-
- Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191(10):1147-1157. doi:10.1164/rccm.201412-2323OC - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

