An Overview of Optic Pathway Glioma With Neurofibromatosis Type 1: Pathogenesis, Risk Factors, and Therapeutic Strategies
- PMID: 38837168
- PMCID: PMC11160950
- DOI: 10.1167/iovs.65.6.8
An Overview of Optic Pathway Glioma With Neurofibromatosis Type 1: Pathogenesis, Risk Factors, and Therapeutic Strategies
Abstract
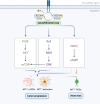
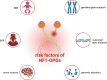
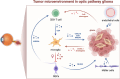
Optic pathway gliomas (OPGs) are most predominant pilocytic astrocytomas, which are typically diagnosed within the first decade of life. The majority of affected children with OPGs also present with neurofibromatosis type 1 (NF1), the most common tumor predisposition syndrome. OPGs in individuals with NF1 primarily affect the optic pathway and lead to visual disturbance. However, it is challenging to assess risk in asymptomatic patients without valid biomarkers. On the other hand, for symptomatic patients, there is still no effective treatment to prevent or recover vision loss. Therefore, this review summarizes current knowledge regarding the pathogenesis of NF1-associated OPGs (NF1-OPGs) from preclinical studies to seek potential prognostic markers and therapeutic targets. First, the loss of the NF1 gene activates 3 distinct Ras effector pathways, including the PI3K/AKT/mTOR pathway, the MEK/ERK pathway, and the cAMP pathway, which mediate glioma tumorigenesis. Meanwhile, non-neoplastic cells from the tumor microenvironment (microglia, T cells, neurons, etc.) also contribute to gliomagenesis via various soluble factors. Subsequently, we investigated potential genetic risk factors, molecularly targeted therapies, and neuroprotective strategies for tumor prevention and vision recovery. Last, potential directions and promising preclinical models of NF1-OPGs are presented for further research. On the whole, NF1-OPGs develop as a result of the interaction between glioma cells and the tumor microenvironment. Developing effective treatments require a better understanding of tumor molecular characteristics, as well as multistage interventions targeting both neoplastic cells and non-neoplastic cells.
Conflict of interest statement
Disclosure:
Figures
Similar articles
-
Neurofibromatosis type 1-associated optic pathway gliomas: pathogenesis and emerging treatments.Eur Rev Med Pharmacol Sci. 2023 Jun;27(12):5636-5653. doi: 10.26355/eurrev_202306_32804. Eur Rev Med Pharmacol Sci. 2023. PMID: 37401302 Clinical Trial.
-
Optic Pathway Gliomas in Neurofibromatosis Type 1.J Child Neurol. 2018 Jan;33(1):73-81. doi: 10.1177/0883073817739509. J Child Neurol. 2018. PMID: 29246098 Free PMC article. Review.
-
Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1.J Neurosci Res. 2019 Jan;97(1):45-56. doi: 10.1002/jnr.24250. Epub 2018 Apr 28. J Neurosci Res. 2019. PMID: 29704429 Free PMC article. Review.
-
NF1 mutation drives neuronal activity-dependent initiation of optic glioma.Nature. 2021 Jun;594(7862):277-282. doi: 10.1038/s41586-021-03580-6. Epub 2021 May 26. Nature. 2021. PMID: 34040258 Free PMC article.
-
Type I neurofibromatosis: a geno-oculo-dermatologic update.Curr Opin Ophthalmol. 2012 Sep;23(5):364-72. doi: 10.1097/ICU.0b013e3283570127. Curr Opin Ophthalmol. 2012. PMID: 22871881 Review.
Cited by
-
Café-Au-Lait Macules in Neurofibromatosis Type 1: Birthmark or Biomarker?Cancers (Basel). 2025 Apr 29;17(9):1490. doi: 10.3390/cancers17091490. Cancers (Basel). 2025. PMID: 40361417 Free PMC article.
-
[Progress and prospects in diagnosis and treatment of neurofibromatosis type 1].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Oct 15;38(10):1166-1170. doi: 10.7507/1002-1892.202407005. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 39433488 Free PMC article. Review. Chinese.
References
-
- Dutton J. Gliomas of the anterior visual pathway. Surv Ophthalmol. 1994; 38(5): 427–452. - PubMed
-
- Louis DN, Perry A, Reifenberger G, et al. .. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (Berl). 2016; 131(6): 803–820. - PubMed
-
- Nicolin G, Parkin P, Mabbott D, et al. .. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer. 2009; 53(7): 1231–1237. - PubMed
-
- Listernick R, Louis D, Packer R, Gutmann D.. Optic pathway gliomas in children with neurofibromatosis. 1. Consensus statement from the NF1 optic pathway glioma task force. Ann Neurol. 1997; 41(2): 143–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

