Metformin for preventing the progression of chronic kidney disease
- PMID: 38837240
- PMCID: PMC11152183
- DOI: 10.1002/14651858.CD013414.pub2
Metformin for preventing the progression of chronic kidney disease
Abstract
Background: Metformin has been used in the management of diabetes for decades. It is an effective, low-cost intervention with a well-established safety profile. Emerging evidence suggests that metformin targets a number of pathways that lead to chronic kidney damage, and long-term use may, therefore, slow the rate of kidney function decline and chronic kidney disease (CKD) progression.
Objectives: To evaluate the effect of metformin therapy on kidney function decline in patients with CKD with or without diabetes mellitus and assess the safety and dose tolerability in this population.
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 19 July 2023 with assistance from an Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: We included randomised controlled trials (RCTs) that reported kidney-related outcomes with a minimum duration of 12 months delivery of the metformin intervention and whose eligibility criteria included adult participants with either i) a diagnosis of CKD of any aetiology and/or ii) those with a diagnosis of diabetes mellitus. Comparisons included placebo, no intervention, non-pharmacological interventions, other antidiabetic medications or any other active control. Studies that included patients on any modality of kidney replacement therapy were excluded.
Data collection and analysis: Two authors independently carried out data extraction using a standard data extraction form. The methodological quality of the included studies was assessed using the Cochrane risk of bias tool. Summary estimates of effect were obtained using a random-effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results: This review included 11 studies reporting on 8449 randomised participants. Studies were conducted in patient populations with Autosomal Dominant Polycystic Kidney Disease (ADPKD) (four studies) or diabetes mellitus (seven studies). Six studies compared metformin with no active control, four studies compared metformin with active controls (rosiglitazone, glyburide, pioglitazone, or glipizide), and one study included treatment arms that randomised to either metformin, diet and lifestyle modifications, or other antidiabetic therapies. The risk of bias in included studies varied; two studies were abstract-only publications and were judged to have a high risk of bias in most domains. Other included publications were judged to have a low risk of bias in most domains. Across comparisons, GRADE evaluations for most outcomes were judged as low or very low certainty, except for those relating to side effects, tolerance, and withdrawals, which were judged as moderate certainty. The evidence suggests that compared to placebo, metformin may result in i) a slightly smaller decline in kidney function (3 studies, 505 participants: MD 1.92 mL/min, 95% CI 0.33 to 3.51; I2 = 0%; low certainty), ii) very uncertain effects on the incidence of kidney failure (1 study, 753 participants: RR 1.20, 95% CI 0.17 to 8.49), iii) little or no effect on death (3 studies, 865 participants: RR 1.00, 95% CI 0.76 to 1.32; I2 = 0%; moderate certainty), iv) little or no effect on the incidence of serious adverse events (3 studies, 576 participants: RR 1.15, 95% CI 0.76 to 1.72; I2 = 0%; moderate certainty), and v) likely higher incidence of intolerance leading to study withdrawal than placebo (4 studies, 646 participants: RR 2.19, 95% CI 1.46 to 3.27; I2 = 0%; moderate certainty). The certainty of the evidence for proteinuria was very uncertain. Compared to other active controls (rosiglitazone, glyburide, pioglitazone, or glipizide), metformin i) demonstrated very uncertain effects on kidney function decline, ii) may result in little or no difference in death (3 studies, 5608 participants: RR 0.95 95% CI 0.63 to 1.43; I2 = 0%; low certainty), iii) probably results in little or no difference in intolerance leading to study withdrawal (3 studies, 5593 participants: RR 0.92, 95% CI, 0.79 to 1.08; I2 = 0%; moderate certainty), iv) probably results in little or no difference in the incidence of serious adverse events (2 studies, 5545 participants: RR 1.16, 95% CI 0.79 to 1.71; I2 = 0%; moderate certainty), and v) may increase the urinary albumin-creatinine ratio (2 studies, 3836 participants: MD 14.61, 95% CI 8.17 to 21.05; I2 = 0%; low certainty). No studies reported the incidence of kidney failure.
Authors' conclusions: This review highlights the lack of RCTs reporting on the effects of metformin on kidney function, particularly in patients with CKD. Future research in this field requires adequately powered RCTs comparing metformin to placebo or standard care in those with CKD. Seven ongoing studies were identified in this review, and future updates, including their findings, may further inform the results of this review.
Trial registration: ClinicalTrials.gov NCT00279045 NCT02903511 NCT01483560 NCT02656017 NCT01794143 NCT01245166 NCT01289119 NCT02252081 NCT03222765 NCT03764605 NCT03831464 NCT04939935 NCT05373680 NCT05469659 NCT03982381.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Ragada El‐Damanawi: No relevant interests were disclosed
Isabelle Kitty Stanley: No relevant interests were disclosed
Christine Staatz: No relevant interests were disclosed
Elaine M Pascoe: No relevant interests were disclosed
Jonathan C Craig: No relevant interests were disclosed
David W Johnson has received consultancy fees, research grants, speaker's honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care. He has received consultancy fees from AstraZeneca and travel sponsorships from Amgen. All funding was unrelated to this review
Andrew J Mallett is a Principle Investigator for a proposed investigator‐initiated clinical trial applying metformin to slow progression of kidney dysfunction in autosomal dominant polycystic kidney disease. In this role, he has received competitive peer‐reviewed research grant funding from PKD Australia for this clinical trial
Carmel M Hawley has received fees from Amgen, Shire, Roche, Abbott, Bayer, Fresenius, Baxter, Gambro, Janssen‐Cilag and Genzyme in relation to consultancy, speakers' fees, education, and grants for activities unrelated to this review
Elasma Milanzi: No relevant interests were disclosed
Thomas F Hiemstra: No relevant interests were disclosed
Andrea K Viecelli: No relevant interests were disclosed
Figures
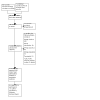
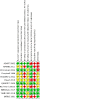





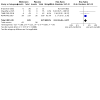
















Update of
References
References to studies included in this review
ADOPT 2002 {published data only}
-
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. New England Journal of Medicine 2006;355(23):2427-43. [PMID: ] - PubMed
-
- Kahn SE, Zinman B, Haffner SM, O'Neill MC, Kravitz BG, Yu D, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes 2006;55(8):2357-64. [MEDLINE: ] - PubMed
-
- Viberti G, Kahn SE, Greene DA, Herman WH, Zinman B, Holman RR, et al. A Diabetes Outcome Progression Trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care 2002;25(10):1737-43. [PMID: ] - PubMed
APRIME 2011 {published data only}UMIN000004367
-
- Morikawa A, Ishizeki K, Iwashima Y, Yokoyama H, Muto E, Oshima E, et al. Pioglitazone reduces urinary albumin excretion in renin-angiotensin system inhibitor-treated type 2 diabetic patients with hypertension and microalbuminuria: the APRIME study. Clinical & Experimental Nephrology 2011;15(6):848–53. [PMID: ] - PubMed
Brosnahan 2022 {published data only}
Campbell 1994 {published data only}
-
- Campbell IW, Menzies DG, Chalmers J, McBain AM, Brown IR. One year comparative trial of metformin and glipizide in type 2 diabetes mellitus. Diabete et Metabolisme 1994;21(4):394-400. [PMID: ] - PubMed
Chaudhary 2021 {published data only}
-
- Roy Chaudhary A, Goswami M, Sen D, Sircar D, Pandey R. POS-494 An Open label randomized controlled study to evaluate the role of Metformin to retard the progression of ADPKD [abstract]. Kidney International Reports 2021;6(4):S213-4. [DOI: 10.1016/j.ekir.2021.03.521] - DOI
Pasari 2019 {published data only}
-
- Pasari A, Balwani M, Trivedi M, Patel M. SUN-200 Role of metformin in ADPKD patients [abstract]. Kidney International Reports 2019;4(7):S242. [DOI: 10.1016/J.EKIR.2019.05.603] - DOI
QUARTET 2004 {published data only}
-
- Charbonnel B, Schernthaner G, Brunetti P, Matthews DR, Urquhart R, Tan MH, et al. Long-term efficacy and tolerability of add-on pioglitazone therapy to failing monotherapy compared with addition of gliclazide or metformin in patients with type 2 diabetes. Diabetologia 2005;48(6):1093-104. [MEDLINE: ] - PubMed
-
- Charbonnel BH, Matthews DR, Schernthaner G, Hanefeld M, Brunetti P. A long-term comparison of pioglitazone and gliclazide in patients with type 2 diabetes mellitus: a randomized, double-blind, parallel-group comparison trial. Diabetic Medicine 2005;22(4):399-405. [MEDLINE: ] - PubMed
-
- Hanefeld M, Brunetti P, Schernthaner GH, Matthews DR, Charbonnel BH. One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care 2004;27(1):141-7. [PMID: ] - PubMed
-
- Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. Journal of Clinical Endocrinology & Metabolism 2004;89(12):6068–76. [PMID: ] - PubMed
Rachmani 2002a {published data only}
-
- Rachmani R, Slavachevski I, Levi Z, Zadok B, Kedar Y, Ravid M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. European Journal of Internal Medicine 2002;13(7):428–33. [PMID: ] - PubMed
REMOVAL 2017 {published data only}
-
- Petrie JR, Chaturvedi N, Ford I, Brouwers MC, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial [Erratum in: Lancet Diabetes Endocrinol. 2017 Aug;5(8):e5] [Erratum in: Lancet Diabetes Endocrinol. 2017 Nov;5(11):e7]. The Lancet Diabetes & Endocrinology 2017;5(8):597–609. [PMID: ] - PMC - PubMed
-
- Petrie JR, Chaturvedi N, Ford I, Hramiak I, Hughes AD, Jenkins AJ, et al. Metformin in adults with type 1 diabetes: design and methods of REducing with MetfOrmin Vascular Adverse Lesions (REMOVAL): an international multicentre trial. Diabetes, Obesity & Metabolism 2017;19(4):509–16. [PMID: ] - PMC - PubMed
-
- Timmons JG, Greenlaw N, Stehouwer CD, Brouwers M, Chaturvedi N, Hughes AD et al. Renal effects of metformin in type 1 diabetes (T1D): the REMOVAL trial [abstract]. Diabetes 2020;69(Suppl 1):490-P. [DOI: 10.2337/db20-490-P] [EMBASE: 633379515] - DOI
-
- Timmons JG, Greenlaw N, Welsh P, Hughes AD, Sattar N, Colhoun HM, et al. Metformin and renal function in type 1 diabetes: the REMOVAL (REducing with MetfOrmin Vascular Adverse Lesions) trial [abstract no: P385]. Diabetic Medicine 2020;37(Suppl 1):142. [EMBASE: 634147394]
TAME‐PKD 2018 {published data only}
-
- Seliger SL, Abebe KZ, Hallows KR, Miskulin D, Perrone RD, Watnick TJ, et al. Design and methods of a randomized controlled trial of metformin in ADPKD (TAME-PKD) [abstract no: PUB388]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):1063. [EMBASE: 633704216]
UKPDS 1991 {published data only}
-
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):854-65. [PMID: ] - PubMed
-
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group [Erratum in: Lancet 1999 Aug 14;354(9178):602]. Lancet 1998;352(9131):837-53. [PMID: ] - PubMed
-
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Annals of Internal Medicine 1996;124(1 Pt 2):136-45. [PMID: ] - PubMed
References to studies excluded from this review
Amador‐Licona 2000 {published data only}
-
- Amador-Licona N, Guizar-Mendoza J, Vargas E, Sanchez-Camargo G, Zamora-Mata L. The short-term effect of a switch from glibenclamide to metformin on blood pressure and microalbuminuria in patients with type 2 diabetes mellitus. Archives of Medical Research 2000;31(6):571-5. [PMID: ] - PubMed
Bayrasheva 2016 {published data only}
-
- Bayrasheva V, Babenko A, Bairamov A, Chefu S, Shatalov I, Grineva E. Nephroprotective properties of metformin in randomized, comparative, prospective clinical study [abstract no: 51]. European Heart Journal 2016;37(Suppl 1):4. [EMBASE: 612282019]
Bayrasheva 2017 {published data only}
-
- Bayrasheva V, Babenko A, Pchelin I, Arefjeva A, Chefu S, Ivanova A, et al. Nephroprotective properties of vildagliptin and metformin combination in randomised, comparative, prospective clinical study [abstract no: 264]. Diabetologia 2017;60(1):S124. [EMBASE: 618052200]
Charbonnel 2005 {published data only}
-
- Charbonnel B, Schernthaner G, Brunetti P, Matthews DR, Urquhart R, Tan MH, et al. Long-term efficacy and tolerability of add-on pioglitazone therapy to failing monotherapy compared with addition of gliclazide or metformin in patients with type 2 diabetes. Diabetologia 2005;48(6):1093-104. [PMID: ] - PubMed
Chen 2006h {published data only}
-
- Chen H, Song QH, Wang ZS, Tan LL. Observation on the therapeutic effect of rosiglitazone combined with metformin on diabetic nephropathy. China Tropical Medicine 2006;6(7):1194-5. [CENTRAL: CN-00783741]
ChiCTR‐IPR‐17012793 {published data only}IPR‐17012793
-
- Effects of metformin on contrast-induced nephropathy in diabetes mellitus after cardiac catheterization procedures. www.chictr.org.cn/showproj.aspx?proj=21884 (accessed 27 April 2024).
Collier 1989 {published data only}
-
- Collier A, Watson H H, Patrick A, Ludlam C, Clarke B. Effect of glycaemic control, metformin and gliclazide on platelet density and aggregability in recently diagnosed type 2 (non-insulin-dependent) diabetic patients. Diabete et Metabolisme 1989;15(6):420-5. [PMID: ] - PubMed
DeFronzo 1995 {published data only}
-
- DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. New England Journal of Medicine 1995;333(9):541-9. [PMID: ] - PubMed
Dornan 1991 {published data only}
-
- Dornan TL, Heller SR, Peck GM, Tattersall RB. Double-blind evaluation of efficacy and tolerability of metformin in NIDDM. Diabetes Care 1991;14(4):342-4. [PMID: ] - PubMed
DPPOS 2009 {published data only}
-
- Kim C, Ricardo A, Boyko EJ, Christophi CA, Temprosa M, Watson K, et al. Sex hormones and development of chronic kidney disease in the diabetes prevention program outcomes study (DPPOS) [abstract]. Diabetes 2018;67(Suppl 1):A421. [EMBASE: 623566488]
EUCTR2011‐004245‐41‐GB {published data only}2011‐004245‐41‐GB
-
- EUCTR2011-004245-41-GB. Study of effects of Glucophage SR (a slow relaese metformin) tablet in patients with type-2 (adult onset) Diabetes and mild kidney dysfunction [Safety, Tolerability and Effectiveness of Glocophage®SR in patients with type-2 Diabetes and Chronic Kidney Disease (eGFR 30 to 45mL/minute/1.73m 2 )]. www.clinicaltrialsregister.eu/ctr-search/search?query=2011-004245-41 (date accessed: 27 April 2024).
GRADE 2023 {published data only}
Grant 1996 {published data only}
-
- Grant PJ. The effects of high- and medium-dose metformin therapy on cardiovascular risk factors in patients with type II diabetes. Diabetes Care 1996;19(1):64-6. [PMID: ] - PubMed
Hällsten 2002 {published data only}
-
- Hallsten K, Virtanen KA, Lonnqvist F, Sipila H, Oksanen A, Viljanen T, et al. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 2002;51(12):3479-85. [PMID: ] - PubMed
Henry 2018 {published data only}
-
- Henry RR, Frias JP, Walsh B, Skare S, Hemming J, Burns C, et al. Improved glycemic control with minimal systemic metformin exposure: effects of Metformin Delayed-Release (Metformin DR) targeting the lower bowel over 16 weeks in a randomized trial in subjects with type 2 diabetes. PLoS ONE [Electronic Resource] 2018;13(9):e0203946. [PMID: ] - PMC - PubMed
Hermann 1994 {published data only}
-
- Hermann LS, Schersten B, Bitzen PO, Kjellstrom T, Lindgarde F, Melander A. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations: a double-blind controlled study. Diabetes Care 1994;17(10):1100-9. [PMID: ] - PubMed
Horton 2000 {published data only}
-
- Horton ES, Clinkingbeard C, Gatlin M, Foley J, Mallows S, Shen S. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care 2000;23(11):1660-5. [PMID: ] - PubMed
IRCT2012102111194N1 {published data only}2012102111194N1
-
- IRCT2012102111194N1. Evaluation of metformin effect on progression of autosomal dominant polycystic kidney disease; a clinical trial. http://en.irct.ir/trial/11501 (date registered: 13 February 2013).
Ji 2016 {published data only}
-
- Ji LN, Pan CY, Lu JM, Li H, Zhu DL, Li Q, et al. Efficacy and safety of combination therapy with vildagliptin and metformin versus metformin uptitration in Chinese patients with type 2 diabetes inadequately controlled with metformin monotherapy: a randomized, open-label, prospective study (VISION). Diabetes, Obesity & Metabolism 2016;18(8):775‐82. [PMID: ] - PubMed
JPRN‐UMIN000003830 {published data only}UMIN000003830
-
- JPRN-UMIN000003830. Effect of pioglitazone and metformin in combination with insulin therapy in patients with type 2 diabetes. https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R0000... (date of registration: 1 July 2010).
NCT01245166 {published data only}
-
- NCT01245166. A phase III randomized, double-blind, parallel-group study to evaluate the efficacy and safety of acarmet (metformin HCl 500 mg plus acarbose 50 mg tablets) versus acarbose alone in subjects with type 2 diabetes mellitus. https://clinicaltrials.gov/study/NCT01245166 (date posted: 22 November 2010).
NCT01289119 {published data only}
-
- NCT01289119. Efficacy and safety of alogliptin in participants with type 2 diabetes [An international, multicenter, randomized, double-blind, placebo-controlled, phase 3 study to determine the efficacy and safety of SYR-322 when used in subjects with type 2 diabetes]. clinicaltrials.gov/study/NCT01289119 (first posted: 3 February 2011).
NCT02252081 {published data only}
-
- NCT02252081. Metformin in kidney disease [Dysmetabolism of chronic kidney disease and vascular health]. https://clinicaltrials.gov/study/NCT02252081 (first posted: 29 September 2014).
Onuchin 2010 {published data only}
-
- Onuchin SG, Elsukova OS, Solov'ev OV, Onuchina L. Capabilities of hypoglycemic therapy in women with decompensated type 2 diabetes mellitus. Terapevticheskii Arkhiv 2010;82(8):34‐41. [PMID: ] - PubMed
Pan 2016 {published data only}
PIOCOMB 2011 {published data only}
-
- Forst T, Hanefeld M, Kleine I, Fuchs W, Pfutzner A. Effect of adding pioglitazone and/or metformin to insulin treatment on blood pressure and renal function in patients with diabetes mellitus type 2-Results from the PIOCOMB-study [abstract no: 1159-P]. Diabetes 2011;60(Suppl 1):A318‐9. [EMBASE: 70628923]
-
- Hanefeld M, Pfutzner A, Forst T, Kleine I, Fuchs W. Double-blind, randomized, multicentre, and active comparator controlled investigation of the effect of pioglitazone, metformin, and the combination of both on cardiovascular risk in patients with type 2 diabetes receiving stable basal insulin therapy: the PIOCOMB study. Cardiovascular Diabetology 2011;10:65. [PMID: ] - PMC - PubMed
-
- Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. International Journal of Clinical Practice 2012;66(5):446-56. [PMID: ] - PubMed
Teupe 1991 {published data only}
-
- Teupe B, Bergis K. Prospective randomized two-years clinical study comparing additional metformin treatment with reducing diet in type 2 diabetes. Diabete et Metabolisme 1991;17(1 Pt 2):213-7. [PMID: ] - PubMed
TRANSDIAB 2017 {published data only}
Walsh 2018 {published data only}
-
- Walsh B, Taylor A, Burns C, Fineman M. Metformin delayed-release (Met DR) exhibits favorable pharmacokinetics potentially mitigating risk of use in CKD 3B/4 [abstract no: 318]. American Journal of Kidney Diseases 2018;71(4):595. [EMBASE: 621595989]
Yang 2014 {published data only}TRC‐08000231
-
- Yang W, Liu J, Shan Z, Tian H, Zhou Z, Ji Q, et al. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, non-inferiority randomised trial [Erratum in: Lancet Diabetes Endocrinol. 2014 Feb;2(2):e4]. The Lancet Diabetes & Endocrinology 2014;2(1):46-55. [PMID: ] - PubMed
Zhu 2007 {published data only}
-
- Zhu K, Yang B Y, Jin Y, Liu W. Metformin treatment of early diabetic nephropathy. Jilin Medical Journal 2007;28(17):1845-6. [CENTRAL: CN-00783630]
References to ongoing studies
ePREDICE 2020 {published data only}
-
- Gabriel R, Boukichou Abdelkader N, Acosta T, Gilis-Januszewska A, Gomez-Huelgas R, Makrilakis K, et al. Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data. PLoS ONE [Electronic Resource] 2020;15(4):e0231196. [DOI: doi: 10.1371/journal.pone.0231196] [PMID: ] - PMC - PubMed
NCT03764605 {published data only}
-
- NCT03764605. Metformin vs tolvaptan for treatment of autosomal dominant polycystic kidney disease (METROPOLIS). clinicaltrials.gov/study/NCT03764605 (first posted: 5 December 2018).
NCT03831464 {published data only}
-
- NCT03831464. Metformin as renoprotector of progressive kidney disease (RenoMet) [Metformin as renoprotector in non-diabetic patients with progressive chronic kidney disease (CKD stages 2, 3A and 3B): a multi-centre, practice-oriented, repurposing, double-blind, placebo-controlled, randomized clinical trial]. clinicaltrials.gov/study/NCT03831464 (first posted: 5 February 2019).
NCT04939935 {published data only}
-
- NCT04939935. Implementation of metformin theraPy to Ease DEcline of kidney function in PKD (IMPEDE-PKD) [Implementation of Metformin theraPy to Ease Decline of Kidney Function in Polycystic Kidney Disease (IMPEDE-PKD): a randomised placebo-controlled trial]. clinicaltrials.gov/ct2/show/NCT04939935 2021.
NCT05373680 {published data only}
-
- NCT05373680. Efficacy and safety of metformin versus empagliflozin on chronic kidney disease progression [Clinical study evaluating the efficacy and safety of metformin versus empagliflozin for halting chronic kidney disease progression]. clinicaltrials.gov/show/NCT05373680 (first posted: 13 May 2022).
NCT05469659 {published data only}
-
- NCT05469659. Effect of Tofogliflozin on UACR Compared to Metformin Hydrochloride in Diabetic Kidney Disease (TRUTH-DKD) [Effect of tofogliflozin on urine albumin-to-creatinine ratio compared to metformin hydrochloride in diabetic kidney disease]. clinicaltrials.gov/show/NCT05469659 (first posted: 22 July 2022).
SMARTEST 2023 {published data only}
-
- Lundqvist MH, Patsoukaki V, Jansson S, Norman H, Granstam E, Svensson MK, et al. Health care registers can be instrumental for endpoint capture in clinical diabetes trials: example of microvascular complications in Swedish patients with type 2 diabetes. Diabetes & Vascular Disease Research 2023;20(3):14791641231179878. [DOI: 10.1177/14791641231179878] [PMID: ] - DOI - PMC - PubMed
Additional references
Bannister 2014
-
- Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes, Obesity & Metabolism 2014;16(11):1165-73. [MEDLINE: ] - PubMed
Bello 2017
Boor 2007
-
- Boor P, Sebekova K, Ostendorf T, Floege J. Treatment targets in renal fibrosis. Nephrology Dialysis Transplantation 2007;22(12):3391-407. [MEDLINE: ] - PubMed
Boor 2010
-
- Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nature Reviews Nephrology 2010;6(11):643-56. [MEDLINE: ] - PubMed
Boyle 2010
-
- Boyle JG, Salt IP, McKay GA. Metformin action on AMP-activated protein kinase: a translational research approach to understanding a potential new therapeutic target. Diabetic Medicine 2010;27(10):1097-106. [MEDLINE: ] - PubMed
Corremans 2018
Crowley 2017
De Broe 2018
-
- De Broe ME, Kajbaf F, Lalau JD. Renoprotective effects of metformin. Nephron 2018;138(4):261-74. [MEDLINE: ] - PubMed
de Jager 2010
DPP Research Group 2012
Gheith 2016
Glosse 2018
-
- Glosse P, Feger M, Mutig K, Chen H, Hirche F, Hasan AA, et al. AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney International 2018;94(3):491-501. [MEDLINE: ] - PubMed
Gong 2012
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] - PubMed
Grgic 2012
Heckman‐Stoddard 2017
Higgins 2003
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hussain 2021
-
- Hussain S, Chand Jamali M, Habib A, Hussain MS, Akhtar M, Najmi AK. Diabetic kidney disease: an overview of prevalence, risk factors, and biomarkers. Clinical Epidemiology & Global Health 2021;9:2-6. [EMBASE: 2006713975]
Inzucchi 2014
Irazabal 2015
-
- Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. Journal of the American Society of Nephrology 2015;26(1):160-72. [PMID: ] - PMC - PubMed
Jabbour 2015
-
- Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgraduate Medicine 2015;123(1):15-23. [MEDLINE: ] - PubMed
Jager 2017
-
- Jager KJ, Fraser SD. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrology Dialysis Transplantation 2017;32(Suppl 2):ii121-8. [MEDLINE: ] - PubMed
Jager 2019
-
- Jager KJ, Kovesdy C, Langham R, Rosenberg M, Vivekan J, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney international 2019;96(5):1048-50. [PMID: ] - PubMed
Jha 2013
-
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382(9888):260-72. [MEDLINE: ] - PubMed
Lalau 2018
-
- Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care 2018;41(3):547-53. [MEDLINE: ] - PubMed
Lazarus 2018
Lee 2013
-
- Lee JH, Kim JH, Kim JS, Chang JW, Kim SB, Park JS, et al. AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. American Journal of Physiology - Renal Physiology 2013;304(6):F686-97. [MEDLINE: ] - PubMed
Levey 2011
-
- Levey AS, Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney International 2011;80(1):17-28. [MEDLINE: ] - PubMed
Levin 2017
-
- Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. The Lancet 2017;390(10105):1888-917. [MEDLINE: ] - PubMed
Liu 2006
-
- Liu Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney International 2006;69(2):213-7. [MEDLINE: ] - PubMed
Liyanage 2015
-
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385(9981):1975-82. [MEDLINE: ] - PubMed
Lu 2013
-
- Lu WR, Defilippi J, Braun A. Unleash metformin: reconsideration of the contraindication in patients with renal impairment. Annals of Pharmacotherapy 2013;47(11):1488-97. [PMID: ] - PubMed
McCreight 2016
Neuen 2017
Panchapakesan 2018
-
- Panchapakesan U, Pollock C. Drug repurposing in kidney disease. Kidney International 2018;94(1):40-8. [MEDLINE: ] - PubMed
Pryor 2015
Ravindran 2016
-
- Ravindran S, Kuruvilla V, Wilbur K, Munusamy S. Nephroprotective effects of metformin in diabetic nephropathy. Journal of Cellular Physiology 2016;232(4):731-42. [MEDLINE: ] - PubMed
Rena 2017
Schünemann 2022a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sharma 2017
-
- Sharma S, Sarnak MJ. Epidemiology: The global burden of reduced GFR: ESRD, CVD and mortality. Nature Publishing Group 2017;13(8):447-8. [PMID: ] - PubMed
Takiar 2011
UKPDS Group 1998
-
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group.[Erratum in: Lancet 1998 Nov 7;352(9139):1558]. Lancet 1998;352(9131):854-65. [MEDLINE: ] - PubMed
Valencia 2017
Viollet 2012
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

