Caspase-8 activity mediates TNFα production and restricts Coxiella burnetii replication during murine macrophage infection
- PMID: 38837340
- PMCID: PMC11238558
- DOI: 10.1128/iai.00053-24
Caspase-8 activity mediates TNFα production and restricts Coxiella burnetii replication during murine macrophage infection
Abstract
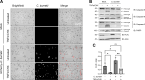
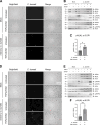
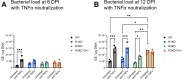
Coxiella burnetii is an obligate intracellular bacteria that causes the global zoonotic disease Q Fever. Treatment options for chronic infection are limited, and the development of novel therapeutic strategies requires a greater understanding of how C. burnetii interacts with immune signaling. Cell death responses are known to be manipulated by C. burnetii, but the role of caspase-8, a central regulator of multiple cell death pathways, has not been investigated. In this research, we studied bacterial manipulation of caspase-8 signaling and the significance of caspase-8 to C. burnetii infection, examining bacterial replication, cell death induction, and cytokine signaling. We measured caspase, RIPK, and MLKL activation in C. burnetii-infected tumor necrosis factor alpha (TNFα)/cycloheximide-treated THP-1 macrophage-like cells and TNFα/ZVAD-treated L929 cells to assess apoptosis and necroptosis signaling. Additionally, we measured C. burnetii replication, cell death, and TNFα induction over 12 days in RIPK1-kinase-dead, RIPK3-kinase-dead, or RIPK3-kinase-dead-caspase-8-/- bone marrow-derived macrophages (BMDMs) to understand the significance of caspase-8 and RIPK1/3 during infection. We found that caspase-8 is inhibited by C. burnetii, coinciding with inhibition of apoptosis and increased susceptibility to necroptosis. Furthermore, C. burnetii replication was increased in BMDMs lacking caspase-8, but not in those lacking RIPK1/3 kinase activity, corresponding with decreased TNFα production and reduced cell death. As TNFα is associated with the control of C. burnetii, this lack of a TNFα response may allow for the unchecked bacterial growth we saw in caspase-8-/- BMDMs. This research identifies and explores caspase-8 as a key regulator of C. burnetii infection, opening novel therapeutic doors.
Keywords: apoptosis; caspases; intracellular bacteria; necroptosis; tumor necrosis factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Caspase-8 activity mediates TNFα production and restricts Coxiella burnetii replication during murine macrophage infection.bioRxiv [Preprint]. 2024 Feb 3:2024.02.02.578698. doi: 10.1101/2024.02.02.578698. bioRxiv. 2024. Update in: Infect Immun. 2024 Jul 11;92(7):e0005324. doi: 10.1128/iai.00053-24. PMID: 38352389 Free PMC article. Updated. Preprint.
Similar articles
-
Caspase-8 activity mediates TNFα production and restricts Coxiella burnetii replication during murine macrophage infection.bioRxiv [Preprint]. 2024 Feb 3:2024.02.02.578698. doi: 10.1101/2024.02.02.578698. bioRxiv. 2024. Update in: Infect Immun. 2024 Jul 11;92(7):e0005324. doi: 10.1128/iai.00053-24. PMID: 38352389 Free PMC article. Updated. Preprint.
-
RIPK1 and RIPK3 regulate TNFα-induced β-cell death in concert with caspase activity.Mol Metab. 2022 Nov;65:101582. doi: 10.1016/j.molmet.2022.101582. Epub 2022 Aug 24. Mol Metab. 2022. PMID: 36030035 Free PMC article.
-
Coxiella burnetii induces apoptosis during early stage infection via a caspase-independent pathway in human monocytic THP-1 cells.PLoS One. 2012;7(1):e30841. doi: 10.1371/journal.pone.0030841. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303462 Free PMC article.
-
Necroptosis in development and diseases.Genes Dev. 2018 Mar 1;32(5-6):327-340. doi: 10.1101/gad.312561.118. Genes Dev. 2018. PMID: 29593066 Free PMC article. Review.
-
Necroptosis and Inflammation.Annu Rev Biochem. 2016 Jun 2;85:743-63. doi: 10.1146/annurev-biochem-060815-014830. Epub 2016 Feb 8. Annu Rev Biochem. 2016. PMID: 26865533 Review.
Cited by
-
CYP1B1-AS1 regulates CYP1B1 to promote Coxiella burnetii pathogenesis by inhibiting ROS and host cell death.Nat Commun. 2025 Aug 13;16(1):7493. doi: 10.1038/s41467-025-62762-2. Nat Commun. 2025. PMID: 40796858 Free PMC article.
-
CYP1B1-AS1 regulates CYP1B1 to promote Coxiella burnetii pathogenesis by inhibiting ROS and host cell death.Res Sq [Preprint]. 2024 Dec 11:rs.3.rs-5390645. doi: 10.21203/rs.3.rs-5390645/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Aug 13;16(1):7493. doi: 10.1038/s41467-025-62762-2. PMID: 39711545 Free PMC article. Updated. Preprint.
-
Role of Type 4B Secretion System Protein, IcmE, in the Pathogenesis of Coxiella burnetii.Pathogens. 2024 May 14;13(5):405. doi: 10.3390/pathogens13050405. Pathogens. 2024. PMID: 38787259 Free PMC article.
-
Rickettsia heilongjiangensis suppresses RIPK1 kinase-mediated host cell death during the infection.Infect Immun. 2025 Aug 12;93(8):e0015825. doi: 10.1128/iai.00158-25. Epub 2025 Jul 3. Infect Immun. 2025. PMID: 40607949 Free PMC article.
References
-
- Davis GE, Cox HR, Parker RR, Dyer RE. 1938. A filter-passing infectious agent isolated from ticks. Public Health Rep (1896-1970) 53:2259. doi:10.2307/4582746 - DOI
-
- Anderson A, Bijlmer H, Fournier P-E, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, Nicholson WL, Paddock CD, Sexton DJ. 2013. Diagnosis and management of Q fever--United States, 2013: recommendations from CDC and the Q fever working group. MMWR Recomm Rep 62:1–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

