Generation of single-round infectious rotavirus with a mutation in the intermediate capsid protein VP6
- PMID: 38837379
- PMCID: PMC11265344
- DOI: 10.1128/jvi.00762-24
Generation of single-round infectious rotavirus with a mutation in the intermediate capsid protein VP6
Abstract
Rotavirus causes severe diarrhea in infants. Although live attenuated rotavirus vaccines are available, vaccine-derived infections have been reported, which warrants development of next-generation rotavirus vaccines. A single-round infectious virus is a promising vaccine platform; however, this platform has not been studied extensively in the context of rotavirus. Here, we aimed to develop a single-round infectious rotavirus by impairing the function of the viral intermediate capsid protein VP6. Recombinant rotaviruses harboring mutations in VP6 were rescued using a reverse genetics system. Mutations were targeted at VP6 residues involved in virion assembly. Although the VP6-mutated rotavirus expressed viral proteins, it did not produce progeny virions in wild-type cells; however, the virus did produce progeny virions in VP6-expressing cells. This indicates that the VP6-mutated rotavirus is a single-round infectious rotavirus. Insertion of a foreign gene, and replacement of the VP7 gene segment with that of human rotavirus clinical isolates, was successful. No infectious virions were detected in mice infected with the single-round infectious rotavirus. Immunizing mice with the single-round infectious rotavirus induced neutralizing antibody titers as high as those induced by wild-type rotavirus. Taken together, the data suggest that this single-round infectious rotavirus has potential as a safe and effective rotavirus vaccine. This system is also applicable for generation of safe and orally administrable viral vectors.IMPORTANCERotavirus, a leading cause of acute gastroenteritis in infants, causes an annual estimated 128,500 infant deaths worldwide. Although live attenuated rotavirus vaccines are available, they are replicable and may cause vaccine-derived infections. Thus, development of safe and effective rotavirus vaccine is important. In this study, we report the development of a single-round infectious rotavirus that can replicate only in cells expressing viral VP6 protein. We demonstrated that (1) the single-round infectious rotavirus did not replicate in wild-type cells or in mice; (2) insertion of foreign genes and replacement of the outer capsid gene were possible; and (3) it was as immunogenic as the wild-type virus. Thus, the mutated virus shows promise as a next-generation rotavirus vaccine. The system is also applicable to orally administrable viral vectors, facilitating development of vaccines against other enteric pathogens.
Keywords: VP6; rotavirus; single-round infection; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

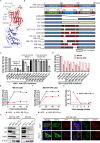
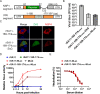


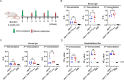
Similar articles
-
Reverse Genetics Approach for Developing Rotavirus Vaccine Candidates Carrying VP4 and VP7 Genes Cloned from Clinical Isolates of Human Rotavirus.J Virol. 2020 Dec 22;95(2):e01374-20. doi: 10.1128/JVI.01374-20. Print 2020 Dec 22. J Virol. 2020. PMID: 33087468 Free PMC article.
-
Targeting of rotavirus VP6 to DEC-205 induces protection against the infection in mice.Vaccine. 2015 Aug 20;33(35):4228-37. doi: 10.1016/j.vaccine.2015.03.080. Epub 2015 Apr 4. Vaccine. 2015. PMID: 25850020
-
Molecular cloning and immunogenicity evaluation of rotavirus structural proteins as candidate vaccine.Int J Biol Macromol. 2013 Aug;59:67-71. doi: 10.1016/j.ijbiomac.2013.04.003. Epub 2013 Apr 12. Int J Biol Macromol. 2013. PMID: 23588003
-
VP6: A candidate rotavirus vaccine.J Infect Dis. 2010 Sep 1;202 Suppl:S101-7. doi: 10.1086/653556. J Infect Dis. 2010. PMID: 20684688 Review.
-
Rotavirus VP6 as a potential vaccine candidate.Rev Med Virol. 2019 Mar;29(2):e2027. doi: 10.1002/rmv.2027. Epub 2019 Jan 6. Rev Med Virol. 2019. PMID: 30614135 Review.
Cited by
-
Rotaviruses and Rotavirus Vaccines: Special Issue Editorial.Viruses. 2024 Oct 24;16(11):1665. doi: 10.3390/v16111665. Viruses. 2024. PMID: 39599780 Free PMC article.
-
A rotavirus VP4 or VP7 monoreassortant panel identifies genotypes that are less susceptible to neutralization by systemic antibodies induced by vaccination or natural infection.mBio. 2025 Jul 9;16(7):e0089725. doi: 10.1128/mbio.00897-25. Epub 2025 May 30. mBio. 2025. PMID: 40444468 Free PMC article.
-
Rotavirus Reverse Genetics Systems and Oral Vaccine Delivery Vectors for Mucosal Vaccination.Microorganisms. 2025 Jul 4;13(7):1579. doi: 10.3390/microorganisms13071579. Microorganisms. 2025. PMID: 40732089 Free PMC article. Review.
References
-
- Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. 2017. Global burden of diarrheal diseases among children in developing countries: incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 35:6783–6789. doi:10.1016/j.vaccine.2017.07.036 - DOI - PubMed
-
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Petri WA, Riddle MS, Steele AD, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC. 2018. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 172:958–965. doi:10.1001/jamapediatrics.2018.1960 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- JP21fk0108122, JP22gm1610008, JP23fk0108668/Japan Agency for Medical Research and Development (AMED)
- JP18H02663, JP21K19379, JP21H02739/MEXT | Japan Science and Technology Agency (JST)
- JP22K07100/MEXT | Japan Science and Technology Agency (JST)
- JPMJMS2025/MEXT | JST | Moonshot Research and Development Program (Moonshot)
- BIKEN Foundation (BIKEN)
LinkOut - more resources
Full Text Sources
Medical

