Bisphosphonates synergistically enhance the antifungal activity of azoles in dermatophytes and other pathogenic molds
- PMID: 38837382
- PMCID: PMC11332346
- DOI: 10.1128/msphere.00248-24
Bisphosphonates synergistically enhance the antifungal activity of azoles in dermatophytes and other pathogenic molds
Abstract
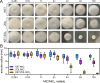
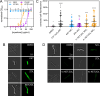
Superficial infections of the skin, hair, and nails by fungal dermatophytes are the most prevalent of human mycoses, and many infections are refractory to treatment. As current treatment options are limited, recent research has explored drug synergy with azoles for dermatophytoses. Bisphosphonates, which are approved to treat osteoporosis, can synergistically enhance the activity of azoles in diverse yeast pathogens but their activity has not been explored in dermatophytes or other molds. Market bisphosphonates risedronate, alendronate, and zoledronate (ZOL) were evaluated for antifungal efficacy and synergy with three azole antifungals: fluconazole (FLC), itraconazole (ITR), and ketoconazole (KET). ZOL was the most active bisphosphonate tested, displaying moderate activity against nine dermatophyte species (MIC range 64-256 µg/mL), and was synergistic with KET in eight of these species. ZOL was also able to synergistically improve the anti-biofilm activity of KET and combining KET and ZOL prevented the development of antifungal resistance. Rescue assays in Trichophyton rubrum revealed that the inhibitory effects of ZOL alone and in combination with KET were due to the inhibition of squalene synthesis. Fluorescence microscopy using membrane- and ROS-sensitive probes demonstrated that ZOL and KET:ZOL compromised membrane structure and induced oxidative stress. Antifungal activity and synergy between bisphosphonates and azoles were also observed in other clinically relevant molds, including species of Aspergillus and Mucor. These findings indicate that repurposing bisphosphonates as antifungals is a promising strategy for revitalising certain azoles as topical antifungals, and that this combination could be fast-tracked for investigation in clinical trials.
Importance: Fungal infections of the skin, hair, and nails, generally grouped together as "tineas" are the most prevalent infectious diseases globally. These infections, caused by fungal species known as dermatophytes, are generally superficial, but can in some cases become aggressive. They are also notoriously difficult to resolve, with few effective treatments and rising levels of drug resistance. Here, we report a potential new treatment that combines azole antifungals with bisphosphonates. Bisphosphonates are approved for the treatment of low bone density diseases, and in fungi they inhibit the biosynthesis of the cell membrane, which is also the target of azoles. Combinations were synergistic across the dermatophyte species and prevented the development of resistance. We extended the study to molds that cause invasive disease, finding synergy in some problematic species. We suggest bisphosphonates could be repurposed as synergents for tinea treatment, and that this combination could be fast-tracked for use in clinical therapy.
Keywords: Trichophyton; antifungal agents; antifungal therapy; azole; bisphosphonate; dermatophytes; drug synergy; ketoconazole; zolendronate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Spectrum of activity and mechanisms of azole-bisphosphonate synergy in pathogenic Candida.Microbiol Spectr. 2024 Jun 4;12(6):e0012124. doi: 10.1128/spectrum.00121-24. Epub 2024 May 2. Microbiol Spectr. 2024. PMID: 38695556 Free PMC article.
-
In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity.Br J Dermatol. 2003 Aug;149(2):296-305. doi: 10.1046/j.1365-2133.2003.05418.x. Br J Dermatol. 2003. PMID: 12932235
-
In Vitro and In Vivo Interactions of TOR Inhibitor AZD8055 and Azoles against Pathogenic Fungi.Microbiol Spectr. 2022 Feb 23;10(1):e0200721. doi: 10.1128/spectrum.02007-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019705 Free PMC article.
-
Antifungal resistance in dermatophytes - review of the epidemiology, diagnostic challenges and treatment strategies for managing Trichophyton indotineae infections.Expert Rev Anti Infect Ther. 2024 Sep;22(9):739-751. doi: 10.1080/14787210.2024.2390629. Epub 2024 Aug 18. Expert Rev Anti Infect Ther. 2024. PMID: 39114868 Review.
-
Antifungal resistance in superficial mycoses.J Dermatolog Treat. 2022 Jun;33(4):1888-1895. doi: 10.1080/09546634.2021.1942421. Epub 2021 Jun 30. J Dermatolog Treat. 2022. PMID: 34132155 Review.
Cited by
-
Uncovering the antifungal potential of Cannabidiol and Cannabidivarin.PLoS Negl Trop Dis. 2025 Jun 5;19(6):e0013081. doi: 10.1371/journal.pntd.0013081. eCollection 2025 Jun. PLoS Negl Trop Dis. 2025. PMID: 40470967 Free PMC article.
-
Targeting fungal lipid synthesis for antifungal drug development and potentiation of contemporary antifungals.NPJ Antimicrob Resist. 2025 Apr 12;3(1):27. doi: 10.1038/s44259-025-00093-4. NPJ Antimicrob Resist. 2025. PMID: 40221522 Free PMC article. Review.
References
-
- de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kupsch C, Stielow JB, Freeke J, Göker M, Rezaei-Matehkolaei A, Mirhendi H, Gräser Y. 2017. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 182:5–31. doi:10.1007/s11046-016-0073-9 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

