DCAF2 regulates the proliferation and differentiation of mouse progenitor spermatogonia by targeting p21 and thymine DNA glycosylase
- PMID: 38837535
- PMCID: PMC11471390
- DOI: 10.1111/cpr.13676
DCAF2 regulates the proliferation and differentiation of mouse progenitor spermatogonia by targeting p21 and thymine DNA glycosylase
Abstract
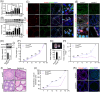
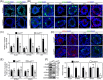
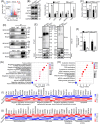
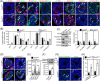
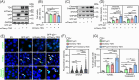
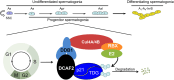
DDB1-Cullin-4-associated factor-2 (DCAF2, also known as DTL or CDT2), a conserved substrate recognition protein of Cullin-RING E3 ligase 4 (CRL4), recognizes and degrades several substrate proteins during the S phase to maintain cell cycle progression and genome stability. Dcaf2 mainly expressed in germ cells of human and mouse. Our study found that Dcaf2 was expressed in mouse spermatogonia and spermatocyte. The depletion of Dcaf2 in germ cells by crossing Dcaf2fl/fl mice with stimulated by retinoic acid gene 8(Stra8)-Cre mice caused a reduction in progenitor spermatogonia and differentiating spermatogonia, eventually leading to the failure of meiosis initiation and male infertility. Further studies showed that depletion of Dcaf2 in germ cells caused abnormal accumulation of the substrate proteins, cyclin-dependent kinase inhibitor 1A (p21) and thymine DNA glycosylase (TDG), decreasing of cell proliferation, increasing of DNA damage and apoptosis. Overexpression of p21 or TDG attenuates proliferation and increases DNA damage and apoptosis in GC-1 cells, which is exacerbated by co-overexpression of p21 and TDG. The findings indicate that DCAF2 maintains the proliferation and differentiation of progenitor spermatogonia by targeting the substrate proteins p21 and TDG during the S phase.
© 2024 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

