Acetate derived from the intestinal tract has a critical role in maintaining skeletal muscle mass and strength in mice
- PMID: 38837588
- PMCID: PMC11150057
- DOI: 10.14814/phy2.16047
Acetate derived from the intestinal tract has a critical role in maintaining skeletal muscle mass and strength in mice
Erratum in
-
Correction to "Acetate derived from the intestinal tract has a critical role in maintaining skeletal muscle mass and strength in mice".Physiol Rep. 2024 Jul;12(13):e16135. doi: 10.14814/phy2.16135. Physiol Rep. 2024. PMID: 38955801 Free PMC article. No abstract available.
Abstract
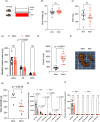
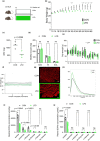
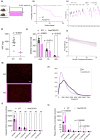
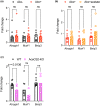
Acetate is a short-chain fatty acid (SCFA) that is produced by microbiota in the intestinal tract. It is an important nutrient for the intestinal epithelium, but also has a high plasma concentration and is used in the various tissues. Acetate is involved in endurance exercise, but its role in resistance exercise remains unclear. To investigate this, mice were administered either multiple antibiotics with and without oral acetate supplementation or fed a low-fiber diet. Antibiotic treatment for 2 weeks significantly reduced grip strength and the cross-sectional area (CSA) of muscle fiber compared with the control group. Intestinal concentrations of SCFAs were reduced in the antibiotic-treated group. Oral administration of acetate with antibiotics prevented antibiotic-induced weakness of skeletal muscle and reduced CSA of muscle fiber. Similarly, a low-fiber diet for 1 year significantly reduced the CSA of muscle fiber and fecal and plasma acetate concentrations. To investigate the role of acetate as an energy source, acetyl-CoA synthase 2 knockout mice were used. These mice had a shorter lifespan, reduced skeletal muscle mass and smaller CSA of muscle fiber than their wild type littermates. In conclusion, acetate derived from the intestinal microbiome can contribute to maintaining skeletal muscle performance.
Keywords: acetate; microbiome; short‐chain fatty acid; skeletal muscle.
© 2024 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Agirman, G. , & Hsiao, E. Y. (2022). Gut microbes shape athletic motivation. Nature, 612(7941), 633–634. - PubMed
-
- Bano, G. , Trevisan, C. , Carraro, S. , Solmi, M. , Luchini, C. , Stubbs, B. , Manzato, E. , Sergi, G. , & Veronese, N. (2017). Inflammation and sarcopenia: A systematic review and meta‐analysis. Maturitas, 96, 10–15. - PubMed
-
- Bernabeu‐Wittel, M. , González‐Molina, Á. , Fernández‐Ojeda, R. , Díez‐Manglano, J. , Salgado, F. , Soto‐Martín, M. , Muniesa, M. , Ollero‐Baturone, M. , & Gómez‐Salgado, J. (2019). Impact of sarcopenia and frailty in a multicenter cohort of Polypathological patients. Journal of Clinical Medicine, 8(4), 535. - PMC - PubMed
-
- Braden, B. , Adams, S. , Duan, L.‐P. , Orth, K.‐H. , Maul, F.‐D. , Lembcke, B. , Hör, G. , & Caspary, W. F. (1995). The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology, 108(4), 1048–1055. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

