Learning, memory and blood-brain barrier pathology in Duchenne muscular dystrophy mice lacking Dp427, or Dp427 and Dp140
- PMID: 38837620
- PMCID: PMC11151035
- DOI: 10.1111/gbb.12895
Learning, memory and blood-brain barrier pathology in Duchenne muscular dystrophy mice lacking Dp427, or Dp427 and Dp140
Abstract
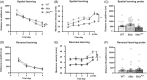
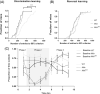
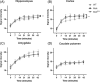
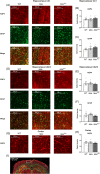
Duchenne muscular dystrophy is a severe neuromuscular disorder that is caused by mutations in the DMD gene, resulting in a disruption of dystrophin production. Next to dystrophin expression in the muscle, different isoforms of the protein are also expressed in the brain and lack of these isoforms leads to cognitive and behavioral deficits in patients. It remains unclear how the loss of the shorter dystrophin isoform Dp140 affects these processes. Using a variety of behavioral tests, we found that mdx and mdx4cv mice (which lack Dp427 or Dp427 + Dp140, respectively) exhibit similar deficits in working memory, movement patterns and blood-brain barrier integrity. Neither model showed deficits in spatial learning and memory, learning flexibility, anxiety or spontaneous behavior, nor did we observe differences in aquaporin 4 and glial fibrillary acidic protein. These results indicate that in contrast to Dp427, Dp140 does not play a crucial role in processes of learning, memory and spontaneous behavior.
Keywords: cognition; dystrophin; neuromuscular disease; spatial learning; spontaneous behavior.
© 2024 The Authors. Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Conflict of interest statement
None related to this work. For full transparency, AAR discloses being employed by LUMC, which has patents on exon skipping technology, some of which has been licensed to BioMarin and subsequently sublicensed to Sarepta. As co‐inventor of some of these patents AAR is entitled to a share of royalties. AAR further discloses being ad hoc consultant for PTC Therapeutics, Sarepta Therapeutics, Regenxbio, Alpha Anomeric, Lilly BioMarin Pharmaceuticals Inc., Eisai, Entrada, Takeda, Splicesense, Galapagos, MitoRx and Astra Zeneca. Past ad hoc consulting has occurred for: CRISPR Therapeutics, Summit PLC, Audentes Santhera, Bridge Bio, Global Guidepoint and GLG consultancy, Grunenthal, Wave and BioClinica. AAR also reports having been a member of the Duchenne Network Steering Committee (BioMarin) and being a member of the scientific advisory boards of Eisai, hybridize therapeutics, silence therapeutics, Sarepta therapeutics. Past SAB memberships: ProQR, Philae Pharmaceuticals. Remuneration for these activities is paid to LUMC. LUMC also received speaker honoraria from PTC Therapeutics, Alnylam Netherlands, Pfizer and BioMarin Pharmaceuticals and funding for contract research from Italfarmaco, Sapreme, Eisai, Galapagos, Synnaffix and Alpha Anomeric. Project funding is received from Sarepta Therapeutics and Entrada.
Figures



References
-
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687‐695. - PubMed
-
- Banihani R, Smile S, Yoon G, et al. Cognitive and neurobehavioral profile in boys with Duchenne muscular dystrophy. J Child Neurol. 2015;30:1472‐1482. - PubMed
-
- Billard C, Gillet P, Signoret J, et al. Cognitive functions in Duchenne muscular dystrophy: a reappraisal and comparison with spinal muscular atrophy. Neuromuscul Disord. 1992;2:371‐378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

